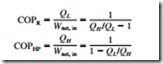SUMMARY
The second law of thermodynamics states that processes occur in a certain direction, not in any direction. A process will not occur unless it satisfies both the first and the second laws of thermodynamics. Bodies that can absorb or reject finite amounts of heat isothermally are called thermal energy reservoirs or heat reservoirs.
Work can be converted to heat directly, but heat can be converted to work only by some devices called heat engines. The thermal efficiency of a heat engine is defined as
where Wnet, out is the net work output of the heat engine, QH is the amount of heat supplied to the engine, and QL is the amount of heat rejected by the engine.
Refrigerators and heat pumps are devices that absorb heat from low-temperature media and reject it to higher-temperature ones. The performance of a refrigerator or a heat pump is expressed in terms of the coefficient of performance, which is defined as
The Kelvin–Planck statement of the second law of thermo- dynamics states that no heat engine can produce a net amount of work while exchanging heat with a single reservoir only.
The Clausius statement of the second law states that no device can transfer heat from a cooler body to a warmer one without leaving an effect on the surroundings.
Any device that violates the first or the second law of thermodynamics is called a perpetual-motion machine.
A process is said to be reversible if both the system and the surroundings can be restored to their original conditions. other process is irreversible. The effects such as friction, non quasi-equilibrium expansion or compression, and heat transfer through a finite temperature difference render a process ir- reversible and are called irreversibilities.
The Carnot cycle is a reversible cycle that is composed of four reversible processes, two isothermal and two adiabatic. The Carnot principles state that the thermal efficiencies of all reversible heat engines operating between the same two reser- voirs are the same, and that no heat engine is more efficient than a reversible one operating between the same two res- ervoirs. These statements form the basis for establishing a thermodynamic temperature scale related to the heat transfers between a reversible device and the high- and low-temperature reservoirs by
Therefore, the QH /QL ratio can be replaced by TH /TL for re- versible devices, where TH and TL are the absolute temperatures of the high- and low-temperature reservoirs, respectively.
A heat engine that operates on the reversible Carnot cycle is called a Carnot heat engine. The thermal efficiency of a Carnot heat engine, as well as all other reversible heat engines, is given by
This is the maximum efficiency a heat engine operating between two reservoirs at temperatures TH and TL can have.
The COPs of reversible refrigerators and heat pumps are given in a similar manner as