■ VAPOR PRESSURE AND CAVITATION
Recall that temperature and pressure are dependent properties for pure sub- stances during phase-change processes, and there is one-to-one correspondence between temperatures and pressures. At a given pressure, the temperature at which a pure substance changes phase is called the saturation temperature, Tsat. Likewise, at a given temperature, the pressure at which a pure substance changes phase is called the saturation pressure, Psat. At an absolute pressure of 1 standard atmosphere (1 atm or 101.325 kPa), for example, the saturation temperature of water is 100°C. Conversely, at a temperature of 100°C, the saturation pressure of water is 1 atm.
Atmospheric air can be viewed as a mixture of dry air and water vapor, and the atmospheric pressure is the sum of the pressure of dry air and the pressure of water vapor. The pressure of a vapor, whether it exists alone or in a mixture with other gases, is called the vapor pressure Pυ. The vapor pressure consti- tutes a small fraction (usually under 3 percent) of the atmospheric pressure since air is mostly nitrogen and oxygen, but the rate of evaporation from open water bodies such as lakes is controlled by the vapor pressure. For example, the saturation pressure of water at 20°C is 2.34 kPa. Therefore, a glass of water at 20°C left in a room at 1 atm will continue evaporating until the pressure of the water vapor in the room rises to 2.34 kPa at which point phase equilibrium is established. This explains why water at 1 atm “evaporates” at temperatures below 100°C.
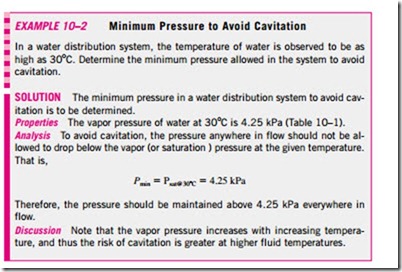
For phase-change processes between the liquid and vapor phases of a pure substance, the saturation pressure and the vapor pressure are equivalent since the vapor is pure. Note that the pressure value would be the same whether it is measured in the vapor or liquid phase (provided that it is measured at a location close to the liquid–vapor interface to avoid the hydrostatic effects). Vapor pressure increases with temperature. Thus, a substance at higher temperatures boils at higher pressures. For example, water boils at 134°C in a pressure cooker operating at 3 atm absolute pressure, but it boils at 93°C in an ordinary pan at a 2000-m elevation, where the atmospheric pressure is 0.8 atm. The saturation (or vapor) pressures are given in Appendices 1 and 2 for various substances. A mini table for water is given in Table 10–1 for easy reference. The reason for our interest in vapor pressure is the possibility of the liquid pressure in liquid-flow systems dropping below the vapor pressure at some locations, and the resulting unplanned vaporization. For example, water at 10°C will flash into vapor and form bubbles at locations (such as the tip regions of impellers or suction sides of pumps) where the pressure drops below 1.23 kPa. The vapor bubbles (called cavitation bubbles since they form “cavities” in the liquid) collapse as they are swept away from the low-pressure regions, generating highly destructive, extremely high-pressure waves. This phenomenon, which is a common cause for drop in performance and even the erosion of impeller blades, is called cavitation, and it is an important consideration in the design of hydraulic turbines and pumps (Fig. 10–10).
Cavitation must be avoided (or at least minimized) in flow systems since it reduces performance, generates annoying vibrations and noise, and causes damage to equipment. The pressure spikes resulting from the large number of bubbles collapsing near a solid surface over a long period of time may cause erosion, surface pitting, fatigue failure, and the eventual destruction of the
Incoming search terms:
- applications of vapour pressupre in fluids and hydraulics in real life
- suitable examples of vapour pressure and cavitation in fluid mechanics and machines
- vapor pressure in fluid mechanics
- vapor pressure sample problems in fluid mechanics
- vapour pressure & cavitation with suitable examples
- vapour pressure and cavitation
- vapour pressure in fluid mechanics
- vapour pressure of liquid fluid mechanics
- द्रव यांत्रिकी में गुहिकायन