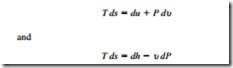SUMMARY
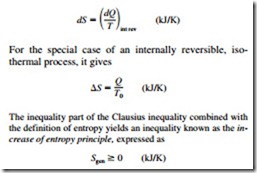
The second law of thermodynamics leads to the definition of a new property called entropy, which is a quantitative measure of microscopic disorder for a system. The definition of entropy is based on the Clausius inequality, given by
![]() where the equality holds for internally or totally reversible processes and the inequality for irreversible processes. Any quantity whose cyclic integral is zero is a property, and entropy is defined as
where the equality holds for internally or totally reversible processes and the inequality for irreversible processes. Any quantity whose cyclic integral is zero is a property, and entropy is defined as
where Sgen is the entropy generated during the process. Entropy change is caused by heat transfer, mass flow, and irreversibilities. Heat transfer to a system increases the entropy, and heat transfer from a system decreases it. The effect of irreversibili- ties is always to increase the entropy.
Entropy is a property, and it can be expressed in terms of more familiar properties through the T ds relations, expressed as
These two relations have many uses in thermodynamics and serve as the starting point in developing entropy-change relations for processes. The successful use of T ds relations de- pends on the availability of property relations. Such relations do not exist for a general pure substance but are available for incompressible substances (solids, liquids) and ideal gases.
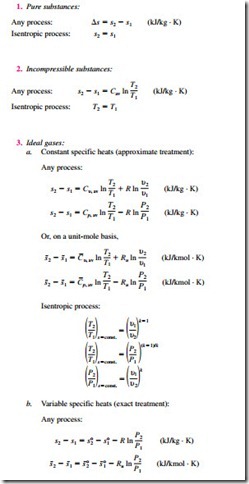
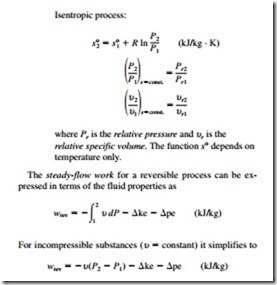
The entropy-change and isentropic relations for a process can be summarized as follows:
The work done during a steady-flow process is proportional to the specific volume. Therefore, u should be kept as small as possible during a compression process to minimize the work input and as large as possible during an expansion process to maximize the work output.
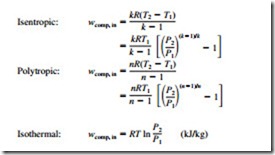
The reversible work inputs to a compressor compressing an ideal gas from T1, P1 to P2 in an isentropic (Puk = constant), polytropic (Pun = constant), or isothermal (Pu = constant) manner, are determined by integration for each case with the following results:
The work input to a compressor can be reduced by using multistage compression with intercooling. For maximum savings from the work input, the pressure ratio across each stage of the compressor must be the same.