SUMMARY
In this chapter, the basic concepts of thermodynamics are introduced and discussed.
A system of fixed mass is called a closed system, or control mass, and a system that involves mass transfer across its boundaries is called an open system, or control volume. The mass-dependent properties of a system are called extensive properties and the others intensive properties. Density is mass per unit volume, and specific volume is volume per unit mass. The sum of all forms of energy of a system is called total energy, which is considered to consist of internal, kinetic, and potential energies. Internal energy represents the molecular energy of a system and may exist in sensible, latent, chemical, and nuclear forms.
A system is said to be in thermodynamic equilibrium if it maintains thermal, mechanical, phase, and chemical equilibrium. Any change from one state to another is called a process. A process with identical end states is called a cycle. During a quasi-static or quasi-equilibrium process, the system remains practically in equilibrium at all times. The state of a simple, compressible system is completely specified by two independent, intensive properties.
The zeroth law of thermodynamics states that two bodies are in thermal equilibrium if both have the same temperature read- ing even if they are not in contact.
The temperature scales used in the SI and the English system today are the Celsius scale and the Fahrenheit scale, respectively. They are related to absolute temperature scales by
The magnitudes of each division of 1 K and 1°C are identical, and so are the magnitudes of each division of 1 R and 1°F. Therefore,
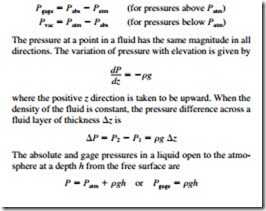
Force exerted by a fluid per unit area is called pressure, and its unit is the pascal, 1 Pa = 1 N/m2. The pressure relative to absolute vacuum is called the absolute pressure, and the differ- ence between the absolute pressure and the local atmospheric pressure is called the gage pressure. Pressures below atmo- spheric pressure are called vacuum pressures. The absolute, gage, and vacuum pressures are related by
Small to moderate pressure differences are measured by a manometer. The pressure in a fluid remains constant in the horizontal direction. Pascal’s principle states that the pressure applied to a confined fluid increases the pressure throughout by the same amount. The atmospheric pressure is measured by a barometer and is given by