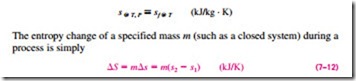ENTROPY CHANGE OF PURE SUBSTANCES
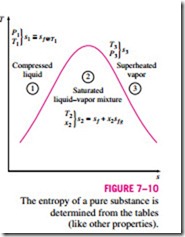
Entropy is a property, and thus the value of entropy of a system is fixed once the state of the system is fixed. Specifying two intensive independent properties fixes the state of a simple compressible system, and thus the value of entropy, as well as the values of other properties at that state. Starting with its defining relation, the entropy change of a substance can be expressed in terms of other properties (see Section 7–7). But in general, these relations are too complicated and are not practical to use for hand calculations. Therefore, using a suitable reference state, the entropies of substances are evaluated from measurable property data following rather involved computations, and the results are tabulated in the same manner as the other properties such as u, u, and h (Fig. 7–10).
The entropy values in the property tables are given relative to an arbitrary reference state. In steam tables the entropy of saturated liquid sf at 0.01°C is assigned the value of zero. For refrigerant-134a, the zero value is assigned to saturated liquid at -40°C. The entropy values become negative at temperatures below the reference value.
The value of entropy at a specified state is determined just like any other property. In the compressed liquid and superheated vapor regions, it can be
obtained directly from the tables at the specified state. In the saturated mixture region, it is determined from
where x is the quality and sf and sfg values are listed in the saturation tables. In the absence of compressed liquid data, the entropy of the compressed liquid can be approximated by the entropy of the saturated liquid at the given temperature:
which is the difference between the entropy values at the final and initial states.
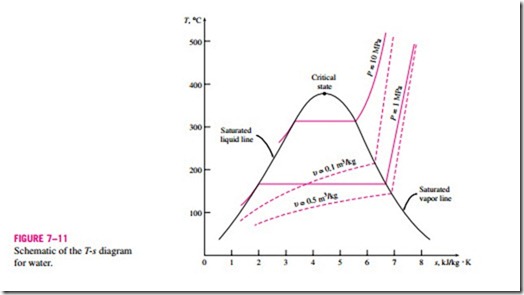
When studying the second-law aspects of processes, entropy is commonly used as a coordinate on diagrams such as the T-s and h-s diagrams. The general characteristics of the T-s diagram of pure substances are shown in Fig. 7–11 using data for water. Notice from this diagram that the constant- volume lines are steeper than the constant-pressure lines and the constant- pressure lines are parallel to the constant-temperature lines in the saturated liquid–vapor mixture region. Also, the constant-pressure lines almost coincide with the saturated liquid line in the compressed liquid region.