Dust risks
Many dusts represent a very significant health hazard. If these materials are to be conveyed it is essential that any dust associated with the material should remain within the conveying system while being transported. If any material is deemed to be toxic to any degree there should be no possibility of any dust being released into the atmosphere. There is also a wide range of materials, which, in a finely divided state, dispersed in air, will propagate a flame through the suspension if ignited.
These materials include foodstuffs such as sugar, flour and cocoa, synthetic materials such as plastics, chemical and pharmaceutical products, metal powders, and fuels such as coal, coke and sawdust. If conveyed with air there is the possibility of a dust explosion within the system. If the dust is released from the system there is the possibility of a dust explosion external to the conveying system.
The potential magnitude of the problem can be illustrated by the fact that during the 17 years from 1962 to 1979 there were 474 recorded dust explosions in the UK, resulting in 25 deaths [1]. This covers the whole area of bulk solids handling, transport and storage and the number of explosions that could be attributed directly to pneumatic conveying systems is not known. In just 2 years (1976 and 1977), dust explosions in grain handling plant in the United States claimed the lives of 87 workers and caused injuries to over 150 more [2]. It is believed that most of these explosions were in bucket elevators and not pneumatic conveying systems, but these statistics highlight the potential for dust explosions, regardless of the source.
Dust emission
Excepting the potentially explosive materials, the most undesirable dusts are those that are so fine that they present a health hazard by remaining suspended in the air for long periods of time. The terminal velocity of a 1 11m particle of silica, for example, is about 1 mm in 30 s, whereas that of a 100 11m particle is about 300 mm/s. The ter- minal velocity of an object depends upon its density, size and shape, and is approximately proportional to the square of its size. Comparative size ranges of some familiar airborne particles are illustrated in Figure 26.1.
Particles falling in the size range of approximately 0.5–5 11m, if inhaled, can reach the lower regions of the lungs where they may be retained. Prolonged exposure to such dusts can cause permanent damage to the lung tissues, symtomized by shortness of breath and increased susceptibility to respiratory infection. Prevention of the emission of these fine particles into the atmosphere is thus of paramount importance. Emissions of larger particles may also give rise to complaints, in a social context, created by the deposition of the particles on neighbouring properties or on vehicles belonging to a company’s own employees.
Dust as a health hazard
When suspended in air the smallest particle visible to the naked eye is about 50–100 11m in diameter, but it is the particles of 0.2–5 11m diameter that are most dangerous for the lungs, as mentioned above. Thus the existence of visible dust gives only indirect evidence of danger, as finer invisible particles will almost certainly be present as well. The fact that no dust can be seen is no reliable indication that dangerous dust may not be present in the air. The large visible particles in a dust cloud will quickly fall to the floor, but it will take many hours for the fine dangerous particles to reach the ground.
Airborne dusts that may be encountered in industrial situations are generally less than about 10 11m in size and can be taken into the body by ingestion, skin absorption or inhalation. The former is rarely a serious problem, but diseases of the skin are of not infrequent occurrence. Allergic reactions are known to be caused by powders contain- ing, for example, metals such as chrome, nickel and cobalt. It is, however, inhalation that presents the greatest hazards for workers in a dusty environment.
Relatively large particles of dust that have been inhaled and become deposited in the respiratory system will usually be carried back to the mouth by cilliary action and be subsequently swallowed or expectorated. At the other extreme, ultra-fine particles (less than about 0.2 11m) that become deposited are likely to pass relatively quickly, generally into solution in the extra-cellular fluids of the lung tissues. Much of this is excreted by the kidneys, either unchanged or after detoxication by the liver. This is the fate of many systemic poisons, for example lead, which gain entry to the body via the lungs [3].
Inhaled particles with the approximate size range of 0.2–5 11m can reach the lower regions of the lungs where they will probably be retained. Prolonged exposure to such dusts can cause various diseases, most of them potentially serious, and often resulting in permanent damage to the lung tissues. The best known are probably the diseases collectively designated ‘pneumoconiosis’ and characterized by chronic fibrosis of the lungs as a result of continuous inhalation of mineral dusts such as silica, asbestos and coal. Generally the symptoms are a chronic shortage of breath and increased susceptibility to respiratory infection. Other dust-related diseases include pneumonitis (an acute inflammation of the lung tissue or bronchioles) and lung cancer.
The relative dangers of some common dusts are compared in Table 26.1 in which the minerals are conveniently classified in Groups I–IV [3].
Dust concentration limits
One of the criteria used in monitoring the compliance of companies with the 1974 Health and Safety at Work Act, and other relevant statutory provisions, is the concentration of airborne dust. The measured concentration is compared with variously defined ‘threshold limit values’ (TLVs) which are also functions of the duration of exposure of personnel to the dust. The most commonly used definitions of threshold limit value are [4]:
• TLV-TWA: time-weighted average concentration for a normal 8 h working day or 40 h week, to which most workers can be repeatedly exposed day after day, without adverse effect.
For further information on actual threshold limit values, Ref [5] should be consulted.
Dust suppression
Where a test for dustiness, or previous experience with a material, indicates that the generation of dust is likely to present a problem, serious consideration should be given to methods of reducing the material’s dustability. It may be appropriate to re-examine the manufacturing process to see if the proportion of fines could be lessened. Agglomeration of the particles, for example by pelletizing, should have a significant effect. If dust is generated during transport, it may be possible to change routing or conveying parameters.
Total enclosure of the processing and handling plant is probably the most desirable approach but, in addition to the high cost, there are obvious problems over accessibility. A generally more satisfactory arrangement is to use some kind of partial enclosure or hood in conjunction with an exhaust system which will draw off the dusty air and so minimize the dispersion of solid particles into the atmosphere. Dusty air collected from a booth, hood or other type of partial or total enclosure must then be filtered, or otherwise cleaned, before it can be released into the atmosphere.
Explosion risks
Apart from choking lungs, irritating eyes and blocking pores, some seemingly innocuous dusts can ignite to cause fires. Many materials, in a dust cloud, can ignite and cause an explosion which could be capable of demolishing a factory. A corn starch powder explosion at General Foods, Banbury did just this in 1981. The company pneumatically conveyed corn starch, used in custard powder production, from a transfer hopper to feed bins, via a diverter valve.
An accumulation of corn starch on the operating cylinder caused a malfunction of this diverter valve. When one hopper was full, the flow should have been diverted to the next one. An already full hopper, therefore, was over-filled causing powder to be dispersed into the surrounding atmosphere. The actual explosion therefore occurred, external to the processing plant where the dust cloud was ignited by electrical arcing from nearby electrical switchgear, burning nine men and blowing out brickwork and windows on all four walls [6].
When an explosible dust cloud is ignited in the open air there is a flash fire but little hazardous pressure develops. If the dust cloud is in a confined situation, however, such as a conveyor or storage vessel, then ignition of the cloud will lead to a build-up of pressure. The magnitude of this pressure depends upon the volume of the suspension, the nature of the material, and the rate of relief to atmosphere. Research has shown that the particle size must be below about 200 11m for a hazard to exist.
At some point in a pneumatic conveying system, or time in the conveying cycle, whether dilute or dense phase, positive or negative pressure, the material will be dispersed as a suspension. A typical point is at discharge into a receiving vessel and a common time is during a transient operation such as start-up or shut-down. Consideration, therefore, must be given to the possibility of an explosion and its effects on the plant should a source of ignition be present.
Due to legal and Health and Safety Executive requirements it is advisable for specialist advice to be sought on dust explosion risks. Authoritative literature on the subject is widely available and there are many tests that can be carried out to determine the seriousness of the problem. It is strongly recommended that a specialist in this field is consulted if there is any doubt about the potential explosion risk connected with pneumatically conveying any material.
Ignition sources
For an explosion to occur two conditions must be satisfied. Firstly, a sufficiently energetic source of ignition must be provided and secondly the concentration of the material in the air must be favourable. Two sources of ignition frequently met in industrial plant are a hot surface and a spark. Consequently, the minimum ignition temperature and the minimum ignition energy are the ignition characteristics commonly measured in routine testing for explosibility.
Ignition temperature, however, is not constant for a given dust cloud, for it depends upon the size and shape of the apparatus used to measure it. Minimum ignition temperatures, therefore, are determined in a standardized form of apparatus, which enables meaningful comparisons between materials to be made. Typical values of minimum ignition temperature for sugar, coffee and cocoa are 350, 410 and 420°C, respectively [8].
The minimum energy relates to ignition by sparks, whether produced be electricity, friction or hot cutting. A characteristic of any form of spark is that a small particle or a small volume of gas at high temperature is produced for a short period of time. Since it is much easier for experimental purposes to measure the energy delivered by an electric spark than by friction or thermal processes, the routine test for determining this characteristic uses an electric spark ignition source. Typical values of minimum igni- tion energy for titanium, polystyrene and coal are 10, 15 and 60 mJ, respectively [7].
Explosibility limits
For a flame to propagate through a dust cloud the concentration of the material in air must fall within a range which is defined by the lower and upper explosibility limits. The lower explosibility limit, or minimum explosible concentration, may be defined as the minimum concentration of material in a cloud or suspension necessary for sustained flame propagation. This is a fairly well defined quantity and can be determined reliably in small scale tests. Values are usually expressed in terms of the mass of material per unit volume of air. Typical values for wood flour and grain dust are 40 and 55 g/m3, respectively [7].
As the concentration of the material is increased above the lower explosibility limit the vigour of the explosion increases. When the dust concentration is increased beyond the stoichiometric value, the dust has a quenching effect. Eventually a con- centration is reached at which flame propagation no longer occurs. This concentration is the upper explosion limit. This limit is not as easy to determine because of the difficulty of achieving a uniform dispersion of the material. From values that have been determined it would appear that for most common materials the upper limit is probably in the range of 2–10 kg/m3. This is equivalent to solids loading ratios of about 1.5–8, which covers the major part of the dilute phase conveying range.
It is reasonable to conclude from this that should a favourable source of ignition be present in a pneumatic conveying system, then dilute phase systems are more of a problem than dense phase systems with respect to explosions. With truly dense phase systems, concentrations are well above the minimum explosibility limit and so it is highly unlikely that an explosion will occur in the pipeline of such a system. Care should still be exercised with such installations, however, since it is possible for an explosive concentration to exist at entry to a cyclone or receiver. Consideration should also be given to the start-up and shut-down transients associated with dense phase systems, for with certain modes of operation dilute phase situations may exist.
Pressure generation
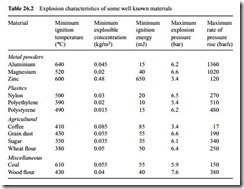
When a dust explosion occurs in industrial plant spectacular destruction can result if it is initially confined in a system that is ultimately too weak to stand the full force of the explosion. Two other important characteristics of a dust explosion therefore are also derived by means of tests. One is the maximum explosion pressure generated, which would be required if it was desired to contain the explosion within the system. The other characteristic is the maximum rate of pressure rise, which would be relevant to the needs of suppressing an explosion within the system. Typical explosion characteristics of some well known materials are presented below in Table 26.2 [7].
The data in the last two columns serves to illustrate the magnitude and rapidity of the sequence of events that follows such an explosion. Explosion pressures may be as
high as 10 bar and the maximum rate of pressure rise may be in excess of 1000 bar/s, which means that it may only take 0.01 s to reach maximum pressure.
If ignition occurs within a pipeline, the pipeline may be capable of withstanding the full explosion pressure. If this is so, the resulting pressure wave would pass along the pipeline and be relieved at the weakest point, which is usually the collection hopper or cyclone. Because of their size these are generally only capable of withstanding pressures of 0.15–0.3 bar gauge and, if exposed to higher internal pressures, may burst or disintegrate. Consequently, the collection unit is likely to be the most vulnerable part of the system.
Expansion effects
The combustion of a dust cloud will result in either a rapid build-up of pressure or in an uncontrolled expansion. It is the expansion effect, or the pressure rise if the expansion is restricted, that presents one of the main hazards in dust explosions. The expansion effects arise principally because of the heat developed in the combustion and, in some cases, to gases being evolved from the dust because of the high temperature to which it has been exposed.
The pressure wave resulting from an uncontrolled dust explosion in a building usually shakes down more dust that has settled over a period of time onto pipe-work, roof beams and supports, ledges, etc. This makes an ideal condition for the secondary explosion that almost always follows. It is this secondary explosion that can demolish a factory and kill the operatives. It is essential, therefore, that an explosion occurring in a pneumatic conveying system is not allowed to be discharged into a building, and that good housekeeping procedures are adopted to minimize the build up of potentially explosive dusts on surfaces in such buildings.
Oxygen concentration
Another characteristic of dust explosions, that can also be measured, is the percentage of oxygen in the conveying gas at which an explosion will occur for a given material. If the oxygen level in air is reduced, a point will be reached at which a flame cannot be supported. If a material is considered to be highly explosive it would generally be conveyed with an inert gas such as nitrogen, instead of air.
For many materials, however, such an extreme measure is not necessary. The use of nitrogen will add significantly to the operating costs, particularly with an open system. If the oxygen content needs to be reduced by only a small amount, a proportion of nitrogen can be added to the air to keep the oxygen level below the required concentration.