We know from experience that a can of cold soda left on a table eventually warms up and that a hot baked potato on the same table cools down (Fig. 4–2). When a body is left in a medium that is at a different temperature, energy transfer takes place between the body and the surrounding medium until thermal equilibrium is established, that is, the body and the medium reach the same temperature. The direction of energy transfer is always from the higher temperature body to the lower temperature one. Once the temperature equal- ity is established, energy transfer stops. In the processes described above, energy is said to be transferred in the form of heat.
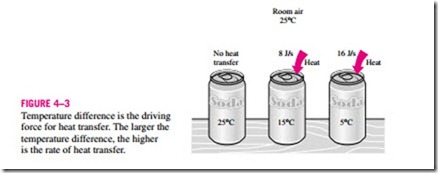
Heat is defined as the form of energy that is transferred between two sys- tems (or a system and its surroundings) by virtue of a temperature difference (Fig. 4–3). That is, an energy interaction is heat only if it takes place because of a temperature difference. Then it follows that there cannot be any heat transfer between two systems that are at the same temperature.
In daily life, we frequently refer to the sensible and latent forms of internal energy as heat, and we talk about the heat content of bodies. In thermo- dynamics, however, we usually refer to those forms of energy as thermal energy to prevent any confusion with heat transfer.
Several phrases in common use today—such as heat flow, heat addition, heat rejection, heat absorption, heat removal, heat gain, heat loss, heat storage, heat generation, electrical heating, resistance heating, frictional heating, gas heating, heat of reaction, liberation of heat, specific heat, sensible heat, latent heat, waste heat, body heat, process heat, heat sink, and heat source—are not consistent with the strict thermodynamic meaning of the term heat, which limits its use to the transfer of thermal energy during a process. However, these phrases are deeply rooted in our vocabulary, and they are used by both ordinary people and scientists without causing any misunderstanding since they are usually interpreted properly instead of being taken literally. (Besides, no acceptable alternatives exist for some of these phrases.) For example, the phrase body heat is understood to mean the thermal energy content of a body. Likewise, heat flow is understood to mean the transfer of thermal energy, not
the flow of a fluidlike substance called heat, although the latter incorrect interpretation, which is based on the caloric theory, is the origin of this phrase. Also, the transfer of heat into a system is frequently referred to as heat addition and the transfer of heat out of a system as heat rejection. Perhaps there are thermodynamic reasons for being so reluctant to replace heat by thermal energy: It takes less time and energy to say, write, and comprehend heat than it does thermal energy.
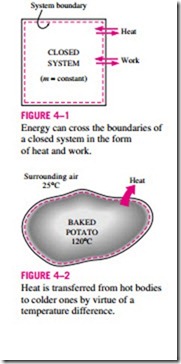
Heat is energy in transition. It is recognized only as it crosses the boundary of a system. Consider the hot baked potato one more time. The potato contains energy, but this energy is heat transfer only as it passes through the skin of the potato (the system boundary) to reach the air, as shown in Fig. 4–4. Once in the surroundings, the transferred heat becomes part of the internal energy of the surroundings. Thus, in thermodynamics, the term heat simply means heat transfer.
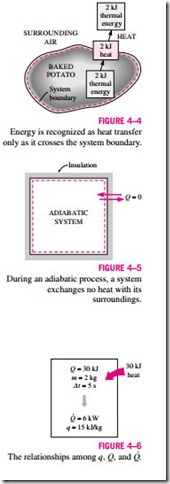
A process during which there is no heat transfer is called an adiabatic process (Fig. 4–5). The word adiabatic comes from the Greek word adiabatos, which means not to be passed. There are two ways a process can be adiabatic: Either the system is well insulated so that only a negligible amount of heat can pass through the boundary, or both the system and the surroundings are at the same temperature and therefore there is no driving force (temperature difference) for heat transfer. An adiabatic process should not be confused with an isothermal process. Even though there is no heat transfer during an adiabatic process, the energy content and thus the temperature of a system can still be changed by other means such as work.
As a form of energy, heat has energy units, kJ (or Btu) being the most common one. The amount of heat transferred during the process between two states (states 1 and 2) is denoted by Q12, or just Q. Heat transfer per unit mass of a system is denoted q and is determined from
Sometimes it is desirable to know the rate of heat transfer (the amount of heat transferred per unit time) instead of the total heat transferred over some time interval (Fig. 4–6). The heat transfer rate is denoted · , where the over-dot stands for the time derivative, or “per unit time.’’ The heat transfer rate Q has the unit kJ/s, which is equivalent to kW. When · varies with time, the amount of heat transfer during a process is determined by integrating Q over the time interval of the process:
Historical Background on Heat
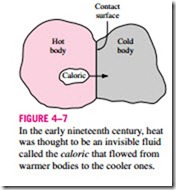
Heat has always been perceived to be something that produces in us a sensation of warmth, and one would think that the nature of heat is one of the first things understood by mankind. However, it was only in the middle of the nineteenth century that we had a true physical understanding of the nature of heat, thanks to the development at that time of the kinetic theory, which treats molecules as tiny balls that are in motion and thus possess kinetic energy. Heat is then defined as the energy associated with the random motion of atoms and molecules. Although it was suggested in the eighteenth and early nineteenth centuries that heat is the manifestation of motion at the molecular level (called the live force), the prevailing view of heat until the middle of the nineteenth century was based on the caloric theory proposed by the French chemist Antoine Lavoisier (1744–1794) in 1789. The caloric theory asserts In the early nineteenth century, heat was thought to be an invisible fluid called the caloric that flowed from warmer bodies to the cooler ones.
that heat is a fluidlike substance called the caloric that is a massless, colorless, odorless, and tasteless substance that can be poured from one body into an- other (Fig. 4–7). When caloric was added to a body, its temperature increased; and when caloric was removed from a body, its temperature decreased. When a body could not contain any more caloric, much the same way as when a glass of water could not dissolve any more salt or sugar, the body was said to be saturated with caloric. This interpretation gave rise to the terms saturated liquid and saturated vapor that are still in use today.
The caloric theory came under attack soon after its introduction. It maintained that heat is a substance that could not be created or destroyed. Yet it was known that heat can be generated indefinitely by rubbing one’s hands together or rubbing two pieces of wood together. In 1798, the American Benjamin Thompson (Count Rumford) (1754–1814) showed in his papers that heat can be generated continuously through friction. The validity of the caloric theory was also challenged by several others. But it was the careful experiments of the Englishman James P. Joule (1818–1889) published in 1843 that finally convinced the skeptics that heat was not a substance after all, and thus put the caloric theory to rest. Although the caloric theory was totally abandoned in the middle of the nineteenth century, it contributed greatly to the development of thermodynamics and heat transfer.
Heat is transferred by three mechanisms: conduction, convection, and radiation. Conduction is the transfer of energy from the more energetic particles of a substance to the adjacent less energetic ones as a result of interaction be- tween particles. Convection is the transfer of energy between a solid surface and the adjacent fluid that is in motion, and it involves the combined effects of conduction and fluid motion. Radiation is the transfer of energy due to the emission of electromagnetic waves (or photons).
Incoming search terms:
- heat is the energy in transition which flows by virtue of
- energy transfer by heat work and mass
- energy transfer that takes place solely because of temperature difference
- heat is the energy
- heat is the energy in transition which flow by virtue of
- its surroundings by virtue of temperature difference
- Heat is transferred between twp system or system and its surrounding by virtue of
- heat is the energy in transition which flows by virtue of ?
- heat is the energy in transition which flow by virtue
- heat is the energy in transition which flo
- heat is the energy in transition whi
- heat is the energy in transition virtue?
- heat is the energy in transition by virtue of
- heat is the e
- heat is a energy in transition which flows by virtue of
- heat energy is the transition which flows by virtue of
- energy ttansfer by heat work amd mass
- why was the caloric theory of heat rejected