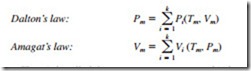SUMMARY
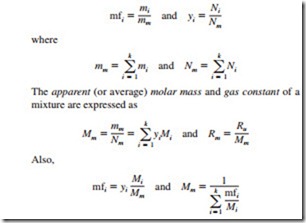
A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. The composition of a gas mixture is described by specifying either the mole fraction or the mass fraction of each component, defined as
Dalton’s law of additive pressures states that the pressure of a gas mixture is equal to the sum of the pressures each gas would exert if it existed alone at the mixture temperature and volume. Amagat’s law of additive volumes states that the volume of a gas mixture is equal to the sum of the volumes each gas would occupy if it existed alone at the mixture temperature and pressure. Dalton’s and Amagat’s laws hold exactly for ideal-gas mixtures, but only approximately for real-gas mixtures. They can be expressed as
Here Pi is called the component pressure and Vi is called the component volume. Also, the ratio Pi /Pm is called the pressure fraction and the ratio Vi /Vm is called the volume fraction of component i. For ideal gases, Pi and Vi can be related to yi by
The quantity yiPm is called the partial pressure and the quantity yiVm is called the partial volume. The P-υ-T behavior of real gas mixtures can be predicted by using generalized compressibility charts. The compressibility factor of the mixture can be expressed in terms of the compressibility factors of the individual gases as
where Zi is determined either at Tm and Vm (Dalton’s law) or at Tm and Pm (Amagat’s law) for each individual gas.
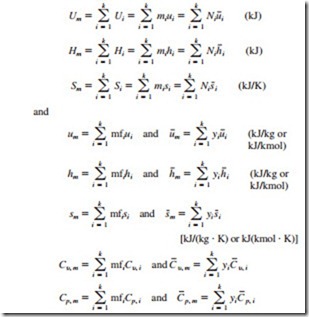
The extensive properties of a gas mixture, in general, can be determined by summing the contributions of each component of the mixture. The evaluation of intensive properties of a gas mixture, however, involves averaging in terms of mass or mole fractions:
These relations are exact for ideal-gas mixtures, and approximate for real-gas mixtures. The properties or property changes of individual components can be determined by using ideal-gas or real-gas relations developed in earlier chapters.
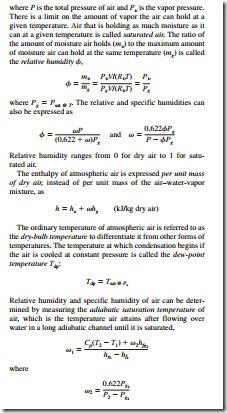
The air in the atmosphere normally contains some water vapor, and it is referred to as atmospheric air. By contrast, air that contains no water vapor is called dry air. In the temperature range encountered in air-conditioning applications, both the dry air and the water vapor can be treated as ideal gases. The enthalpy change of dry air during a process can be determined from
The enthalpy of water vapor in the air can be taken to be equal to the enthalpy of the saturated vapor at the same temperature:
and T2 is the adiabatic saturation temperature. A more practical approach in air-conditioning applications is to use a thermometer whose bulb is covered with a cotton wick saturated with water and to blow air over the wick. The temperature measured in this manner is called the wet-bulb temperature Twb, and it is used in place of the adiabatic saturation temperature. The properties of atmospheric air at a specified total pressure are presented in the form of easily readable charts, called psychrometric charts. The lines of constant enthalpy and the lines of constant wet-bulb temperature are very nearly parallel on these charts.
Most air-conditioning processes can be modeled as steady- flow processes, and therefore they can be analyzed by applying