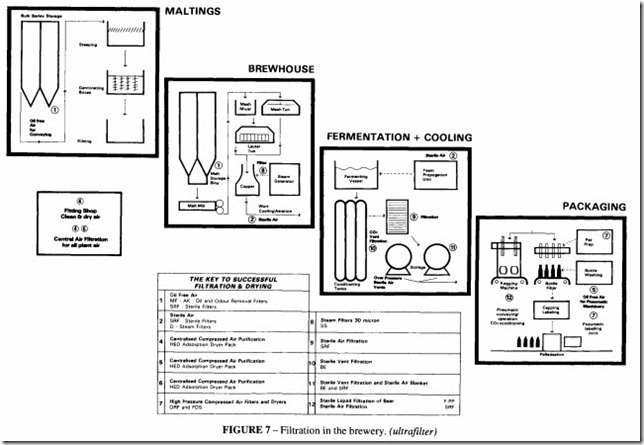
Steam sterilization
Routine sterilization is a requirement for any system using a sterile filter. It is often carried out between batches or in a continuous process plant at prescribed intervals. In batch
operation provided that the filter is kept pressurised and a small air bleed is allowed to flow continuously, several batches can pass before sterilization is carried out. The steam used must be saturated and free from additives or contamination, which implies its own sterile filter, see Figure 5. Steam sterilization is shown in the lower diagram of Figure 3.
A well designed system should last for 12 months before cartridge replacement is
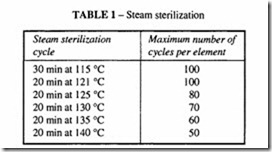
necessary; this probably means of the order of 100 sterilization cycles (according to the recommendations of the manufacturer). Typical cycles are shown in Table 1.The cost of replacement cartridges is small compared with the cost of a failure of the batch due to contamination, so replacement times should not be extended beyond 12 months.
Validation
Nothing should be left to chance when clean sterile compressed air is required for such applications as fermentation, genetic engineering, or pharmaceutical production. It is
essential that any filter used has been tested for integrity to ensure that it will fulfil its designed duty. The best way of testing for integrity is by a cloud of test particles in the critical rangeO.l to 0.3 micron. The test is based on creating an aerosol ofDioctyl Phthalate (DOP) in the critical particle range, challenging the filter with this and measuring any penetration with an aerosol photometer, downstream of the filter. DOP has been replaced by other substances because of possible health hazards and so corn oil or other oils are used giving the same type of particle spread when atomized. This type of validation test is carried out by the manufacturers and is not normally a procedure used by the end user