THERMODYNAMICS
Thermodynamics can be defined as the science of energy. Although every- body has a feeling of what energy is, it is difficult to give a precise definition for it. Energy can be viewed as the ability to cause changes.
The name thermodynamics stems from the Greek words therme (heat) and dynamis (power), which is most descriptive of the early efforts to convert heat into power. Today the same name is broadly interpreted to include all aspects of energy and energy transformations, including power production, refrigeration, and relationships among the properties of matter.
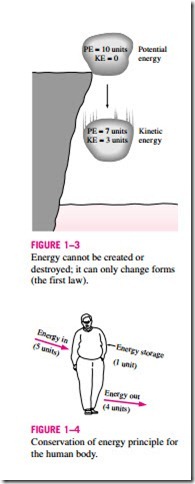
One of the most fundamental laws of nature is the conservation of energy principle. It simply states that during an interaction, energy can change from one form to another but the total amount of energy remains constant. That is, energy cannot be created or destroyed. A rock falling off a cliff, for example, picks up speed as a result of its potential energy being converted to kinetic en- ergy (Fig. 1–3). The conservation of energy principle also forms the backbone of the diet industry: a person who has a greater energy input (food and drinks) than energy output (exercise and metabolism with environmental conditions) will gain weight (store energy in the form of tissue and fat), and a person who has a smaller energy input than output will lose weight (Fig. 1–4). The change in the energy content of a body or any other system is equal to the difference between the energy input and the energy output, and the energy balance is ex- pressed as Ein – Eout = LE.
The first law of thermodynamics is simply an expression of the conservation of energy principle, and it asserts that energy is a thermodynamic property. The second law of thermodynamics asserts that energy has quality as well as quantity, and actual processes occur in the direction of decreasing quality of energy. For example, a cup of hot coffee left on a table eventually cools to room temperature, but a cup of cool coffee in the same room never gets hot by itself. The high-temperature energy of the coffee is degraded (transformed into a less useful form at a lower temperature) once it is transferred to the surrounding air.
Although the principles of thermodynamics have been in existence since the creation of the universe, thermodynamics did not emerge as a science until the construction of the first successful atmospheric steam engines in England by Thomas Savery in 1697 and Thomas Newcomen in 1712. These engines were very slow and inefficient, but they opened the way for the development of a new science. The first and second laws of thermodynamics emerged simultaneously in the 1850s, primarily out of the works of William Rankine, Rudolph Clausius, and Lord Kelvin (formerly William Thomson). The term thermodynamics was first used in a publication by Lord Kelvin in 1849. The first thermodynamic textbook was written in 1859 by William Rankine, a professor at the University of Glasgow.
It is well known that a substance consists of a large number of particles called molecules. The properties of the substance naturally depend on the behavior of these particles. For example, the pressure of a gas in a container is the result of momentum transfer between the molecules and the walls of the container. But one does not need to know the behavior of the gas particles to determine the pressure in the container. It would be sufficient to attach a pressure gage to the container. This macroscopic approach to the study of thermo- dynamics that does not require a knowledge of the behavior of individual particles is called classical thermodynamics. It provides a direct and easy way to the solution of engineering problems. A more elaborate approach, based on the average behavior of large groups of individual particles, is called statistical thermodynamics. This microscopic approach is rather involved and is used in this text only in the supporting role.