Characteristics of hydraulic fluids
In the first chapter of this book, we have examined in detail, the various properties of hydraulic fluids that help determine the system performance and efficiency. There are two other important characteristics, which also play an important role in the life of a hydraulic fluid. These are:
1. Oxidation and corrosion prevention
2. Neutralization number.
Oxidation and corrosion prevention
Oxidation is that process resulting from the chemical reaction of oxygen in the air with oil. This can reduce the life of a hydraulic fluid drastically. Petroleum oils are particularly susceptible to oxidation because oxygen readily unites with both carbon and hydrogen molecules. Most products of oxidation are soluble in oil as well as acidic in nature and can cause severe damage to system components by way of corrosion. The products of oxygen include insoluble gums, sludge and varnish and these tend to increase the viscosity of oil.
There are a number of parameters, which hasten the rate of oxidation once it begins, some of the important ones being heat, pressure, contaminants, water and metal surfaces.
However oxidation is most affected by temperature. Various additives are incorporated in hydraulic oils to inhibit the rate of oxidation. As additives increase the cost of the oil, they should be specified only if necessary, based on the temperature and other environmental conditions.
The relative changes in oil property under oxidizing conditions can be studied with the
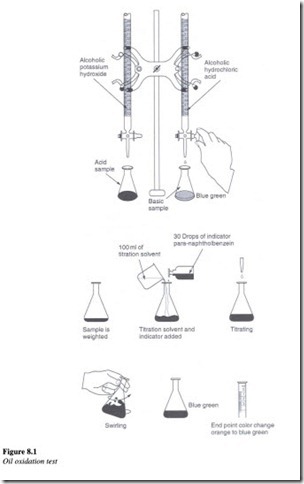
The main purpose behind this test is to measure the resistance to oxidation by measuring the change in the acidity of the oil due to absorbed oxygen. The test procedure is as follows:
A 300 ml sample of oil is placed in a tube and immersed in an oil bath at 95 oc. Three liters per hour of oxygen is allowed to pass continuously through the sample for a period of about 1000 h. The acidity of oil is then measured by determining the neutralization number.
To measure the neutralization number a weighed amount of a sample of oil is placed ina beaker. About 100 ml of titration solvent is added to the sample in the beaker. Further around 30 drops of an indicator is added to this solution. For carrying out the titration process, standard alcoholic potassium hydroxide is added to the solution drop by drop, until the color of the solution changes from orange to blue-green. The amount of potassium hydroxide required in milligrams, indicates the level of oxidation that has taken place. This is the amount needed to neutralize the acid in one gram of oil.
Rust and corrosion are two altogether different phenomena, although they both contaminate the oil and promote wear. Rust is the chemical reaction between iron or steel and oxygen. The presence of moisture in the hydraulic system provides the necessary oxygen. One primary source of oxygen is the atmospheric air, which enters the reservoir through the breather cap.
Corrosion on the other hand, is the chemical reaction between a metal and an acid.
Because of rusting or corrosion, the metal surfaces of the hydraulic components are eaten away. This results in excessive leakage through the affected parts like seals. Rust and corrosion can be resisted by additives, which form a protective layer on the metal surfaces and thereby prevent the occurrence of a chemical reaction.