■ ENERGY BALANCE FOR CLOSED SYSTEMS
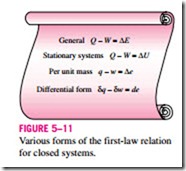
The energy balance (or the first-law) relations already given are intuitive in nature and are easy to use when the magnitudes and directions of heat and work transfers are known. However, when performing a general analytical study or solving a problem that involves an unknown heat or work interaction, we need to assume a direction for the heat or work interactions. In such cases, it is common practice to use the classical thermodynamics sign convention and to assume heat to be transferred into the system (heat input) in the amount of Q and work to be done by the system (work output) in the amount of W, and then to solve the problem. The energy balance relation in that case for a closed system becomes
where Q = Qnet, in = Qin – Qout is the net heat input and W = Wnet, out = Wout – Win is the net work output. Obtaining a negative quantity for Q or W simply means that the assumed direction for that quantity is wrong and should be reversed. Various forms of this “traditional” first-law relation for closed systems are given in Fig. 5–11.
The first law cannot be proven mathematically, but no process in nature is known to have violated the first law, and this should be taken as sufficient proof. Note that if it were possible to prove the first law on the basis of other physical principles, the first law then would be a consequence of those principles instead of being a fundamental physical law itself.
As energy quantities, heat and work are not that different, and you probably wonder why we keep distinguishing them. After all, the change in the energy content of a system is equal to the amount of energy that crosses the system boundaries, and it makes no difference whether the energy crosses the boundary as heat or work. It seems as if the first-law relations would be much simpler if we had just one quantity that we could call energy interaction to represent both heat and work. Well, from the first-law point of view, heat and work are not different at all. From the second-law point of view, however, heat and work are very different, as is discussed in later chapters.