PHASES OF A PURE SUBSTANCE
We all know from experience that substances exist in different phases. At room temperature and pressure, copper is a solid, mercury is a liquid, and nitrogen is a gas. Under different conditions, each may appear in a different phase. Even though there are three principal phases—solid, liquid, and gas— a substance may have several phases within a principal phase, each with a different molecular structure. Carbon, for example, may exist as graphite or diamond in the solid phase. Helium has two liquid phases; iron has three solid phases. Ice may exist at seven different phases at high pressures. A phase is identified as having a distinct molecular arrangement that is homogeneous throughout and separated from the others by easily identifiable boundary surfaces. The two phases of H2O in iced water represent a good example of this. When studying phases or phase changes in thermodynamics, one does not need to be concerned with the molecular structure and behavior of different phases. However, it is very helpful to have some understanding of the molec- ular phenomena involved in each phase, and a brief discussion of phase trans-formations follows.
Intermolecular bonds are strongest in solids and weakest in gases. One reason is that molecules in solids are closely packed together, whereas in gases they are separated by relatively large distances.
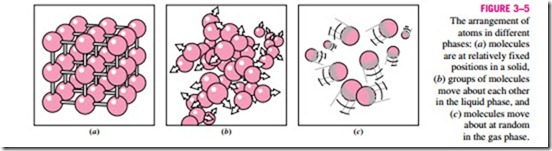
The molecules in a solid are arranged in a three-dimensional pattern (lattice) that is repeated throughout (Fig. 3–3). Because of the small distances be- tween molecules in a solid, the attractive forces of molecules on each other are large and keep the molecules at fixed positions (Fig. 3–4). Note that the at- tractive forces between molecules turn to repulsive forces as the distance be- tween the molecules approaches zero, thus preventing the molecules from piling up on top of each other. Even though the molecules in a solid cannot move relative to each other, they continually oscillate about their equilibrium
positions. The velocity of the molecules during these oscillations depends on the temperature. At sufficiently high temperatures, the velocity (and thus the momentum) of the molecules may reach a point where the intermolecular forces are partially overcome and groups of molecules break away (Fig. 3–5). This is the beginning of the melting process.
The molecular spacing in the liquid phase is not much different from that of the solid phase, except the molecules are no longer at fixed positions relative to each other and they can rotate and translate freely. In a liquid, the inter- molecular forces are weaker relative to solids, but still relatively strong com- pared with gases. The distances between molecules generally experience a slight increase as a solid turns liquid, with water being a notable exception. In the gas phase, the molecules are far apart from each other, and a molecular order is nonexistent. Gas molecules move about at random, continually colliding with each other and the walls of the container they are in. Particularly at low densities, the intermolecular forces are very small, and collisions are the only mode of interaction between the molecules. Molecules in the gas phase are at a considerably higher energy level than they are in the liquid or solid phases. Therefore, the gas must release a large amount of its energy before it can condense or freeze.