■ THE PSYCHROMETRIC CHART
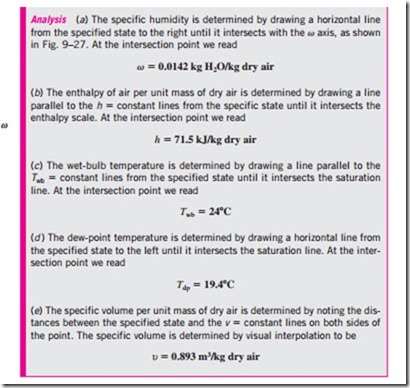
The state of the atmospheric air at a specified pressure is completely specified by two independent intensive properties. The rest of the properties can be calculated easily from the relations already given. The sizing of a typical air-conditioning system involves numerous such calculations, which may eventually get on the nerves of even the most patient engineers. Therefore, there is clear motivation to computerize calculations or to do these calcula- tions once and to present the data in the form of easily readable charts. Such charts are called psychrometric charts, and they are used extensively in air- conditioning applications. A psychrometric chart for a pressure of 1 atm (101.325 kPa or 14.696 psia) is given in Fig. A–33 in SI units and in Fig. A–33E in English units. Psychrometric charts at other pressures (for use at considerably higher elevations than sea level) are also available.
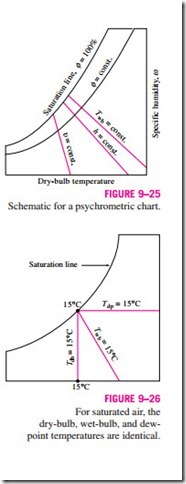
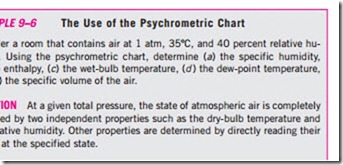
The basic features of the psychrometric chart are illustrated in Fig. 9–25. The dry-bulb temperatures are shown on the horizontal axis, and the specific humidity is shown on the vertical axis. (Some charts also show the vapor pressure on the vertical axis since at a fixed total pressure P there is a one-to-one correspondence between the specific humidity v and the vapor pressure Pυ, as can be seen from Eq. 9–29.) On the left end of the chart, there is a curve (called the saturation line) instead of a straight line. All the saturated air states are located on this curve. Therefore, it is also the curve of 100 percent relative humidity. Other constant relative-humidity curves have the same general shape. Lines of constant wet-bulb temperature have a downhill appearance to the right. Lines of constant specific volume (in m3/kg dry air) look similar, except they are steeper. Lines of constant enthalpy (in kJ/kg dry air) lie very nearly parallel to the lines of constant wet-bulb temperature. Therefore, the constant- wet-bulb-temperature lines are used as constant-enthalpy lines in some charts. For saturated air, the dry-bulb, wet-bulb, and dew-point temperatures are identical (Fig. 9–26). Therefore, the dew-point temperature of atmospheric air at any point on the chart can be determined by drawing a horizontal line (a line of v = constant or Pυ = constant) from the point to the saturated curve. The temperature value at the intersection point is the dew-point temperature. The psychrometric chart also serves as a valuable aid in visualizing the air- conditioning processes. An ordinary heating or cooling process, for example, will appear as a horizontal line on this chart if no humidification or dehumidification is involved (that is, v = constant). Any deviation from a horizontal line indicates that moisture is added or removed from the air during the process.