In this chapter, we deal with nonreacting gas mixtures. A nonreacting gas mixture can be treated as a pure substance since it is usually a homogeneous mixture of different gases. The properties of a gas mixture obviously will de- pend on the properties of the individual gases (called components or constituents) as well as on the amount of each gas in the mixture. Therefore, it is possible to prepare tables of properties for mixtures. This has been done for common mixtures such as air. It is not practical to prepare property tables for every conceivable mixture composition, however, since the number of possible compositions is endless. Therefore, we need to develop rules for deter- mining mixture properties from a knowledge of mixture composition and the properties of the individual components. We do this first for ideal-gas mixtures and then for real-gas mixtures. The basic principles involved are also applicable to liquid or solid mixtures, called solutions.
At temperatures below the critical temperature, the gas phase of a substance is frequently referred to as a vapor. The term vapor implies a gaseous state that is close to the saturation region of the substance, raising the possibility of condensation during a process.
When we deal with a gas–vapor mixture, the vapor may condense out of the mixture during a process, forming a two-phase mixture. This may complicate the analysis considerably. Therefore, a gas–vapor mixture needs to be treated differently from an ordinary gas mixture.
Several gas–vapor mixtures are encountered in engineering. In this chapter, we consider the air–water-vapor mixture, which is the most commonly en- countered gas–vapor mixture in practice. We also discuss air-conditioning, which is the primary application area of air–water-vapor mixtures.
■ COMPOSITION OF A GAS MIXTURE: MASS AND MOLE FRACTIONS
To determine the properties of a mixture, we need to know the composition of the mixture as well as the properties of the individual components. There are two ways to describe the composition of a mixture: either by specifying the number of moles of each component, called molar analysis, or by specifying The mass of a mixture is equal to the sum of the masses of its components.
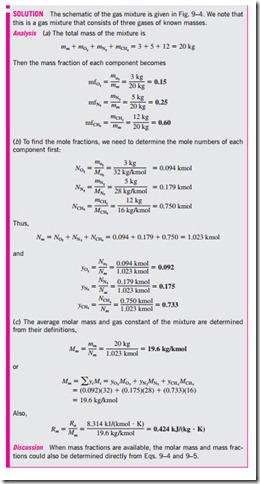
Consider a gas mixture composed of k components. The mass of the mixture mm is the sum of the masses of the individual components, and the mole number of the mixture Nm is the sum of the mole numbers of the individual components* (Figs. 9–1 and 9–2). That is,