ENERGY AND ENVIRONMENT
The conversion of energy from one form to another often affects the environment and the air we breathe in many ways, and thus the study of energy is not complete without considering its impact on the environment (Fig. 2–25). Fossil fuels such as coal, oil, and natural gas have been powering the industrial development and the amenities of modern life that we enjoy since the 1700s, but this has not been without any undesirable side effects. From the soil we farm and the water we drink to the air we breathe, the environment has been paying a heavy toll for it. Pollutants emitted during the combustion of fossil fuels are responsible for smog, acid rain, and global warming and cli- mate change. The environmental pollution has reached such high levels that it became a serious threat to vegetation, wild life, and human health. Air pollution has been the cause of numerous health problems including asthma and cancer. It is estimated that over 60,000 people in the United States alone die each year due to heart and lung diseases related to air pollution.
Hundreds of elements and compounds such as benzene and formaldehyde are known to be emitted during the combustion of coal, oil, natural gas, and wood in electric power plants, engines of vehicles, furnaces, and even fire- places. Some compounds are added to liquid fuels for various reasons (such as MTBE to raise the octane number of the fuel and also to oxygenate the fuel in winter months to reduce urban smog). The largest source of air pollution is the motor vehicles, and the pollutants released by the vehicles are usually grouped as hydrocarbons (HC), nitrogen oxides (NOx), and carbon monoxide (CO) (Fig. 2–26). The HC emissions are a large component of volatile organic com- pounds (VOC) emissions, and the two terms are generally used interchange- ably for motor vehicle emissions. A significant portion of the VOC or HC emissions are caused by the evaporation of fuels during refueling or spillage during spitback or by evaporation from gas tanks with faulty caps that do not close tightly. The solvents, propellants, and household cleaning products that contain benzene, butane, or other HC products are also significant sources of HC emissions.
The increase of environmental pollution at alarming rates and the rising awareness of its dangers made it necessary to control it by legislation and international treaties. In the United States, the Clean Air Act of 1970 (whose passage was aided by the 14-day smog alert in Washington that year) set limits on pollutants emitted by large plants and vehicles. These early standards focused on emissions of hydrocarbons, nitrogen oxides, and carbon monoxide. The new cars were required to have catalytic converters in their exhaust systems to reduce HC and CO emissions. As a side benefit, the removal of lead from gasoline to permit the use of catalytic converters led to a significant reduction in toxic lead emissions.
Emission limits for HC, NOx, and CO from cars have been declining steadily since 1970. The Clean Air Act of 1990 made the requirements on emissions even tougher, primarily for ozone, CO, nitrogen dioxide, and par- ticulate matter (PM). As a result, today’s industrial facilities and vehicles emit a fraction of the pollutants they used to emit a few decades ago. The HC emis- sions of cars, for example, decreased from about 8 gpm (grams per mile) in 1970 to 0.4 gpm in 1980 and about 0.1 gpm in 1999. This is a significant re- duction since many of the gaseous toxics from motor vehicles and liquid fuels are hydrocarbons.
Children are most susceptible to the damages caused by air pollutants since their organs are still developing. They are also exposed to more pollution since they are more active, and thus they breathe faster. People with heart and lung problems, especially those with asthma, are most affected by air pollutants. This becomes apparent when the air pollution levels in their neighbor- hoods rise to high levels.
Ozone and Smog
If you live in a metropolitan area such as Los Angeles, you are probably familiar with urban smog—the dark yellow or brown haze that builds up in a large stagnant air mass and hangs over populated areas on calm hot summer days. Smog is made up mostly of ground-level ozone (O3), but it also contains numerous other chemicals, including carbon monoxide (CO), particulate matter such as soot and dust, volatile organic compounds (VOC) such as benzene, butane, and other hydrocarbons. The harmful ground-level ozone should not be confused with the useful ozone layer high in the stratosphere that protects the earth from the sun’s harmful ultraviolet rays. Ozone at ground level is a pollutant with several adverse health effects.
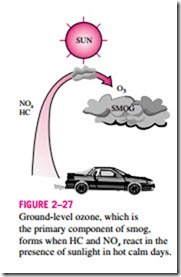
The primary source of both nitrogen oxides and hydrocarbons is the motor vehicles. Hydrocarbons and nitrogen oxides react in the presence of sunlight on hot calm days to form ground-level ozone, which is the primary component of smog (Fig. 2–27). The smog formation usually peaks in late afternoons when the temperatures are highest and there is plenty of sunlight. Although ground-
level smog and ozone form in urban areas with heavy traffic or industry, the prevailing winds can transport them several hundred miles to other cities. This shows that pollution knows of no boundaries, and it is a global problem.
Ozone irritates eyes and damages the air sacs in the lungs where oxygen and carbon dioxide are exchanged, causing eventual hardening of this soft and spongy tissue. It also causes shortness of breath, wheezing, fatigue, head- aches, and nausea, and aggravates respiratory problems such as asthma. Every exposure to ozone does a little damage to the lungs, just like cigarette smoke, eventually reducing the individual’s lung capacity. Staying indoors and minimizing physical activity during heavy smog will minimize damage. Ozone also harms vegetation by damaging leaf tissues. To improve the air quality in areas with the worst ozone problems, reformulated gasoline (RFG) that con- tains at least 2 percent oxygen was introduced. The use of RFG has resulted in significant reduction in the emission of ozone and other pollutants, and its use is mandatory in many smog-prone areas.
The other serious pollutant in smog is carbon monoxide, which is a color- less, odorless, poisonous gas. It is mostly emitted by motor vehicles, and it can build to dangerous levels in areas with heavy congested traffic. It deprives the body’s organs from getting enough oxygen by binding with the red blood cells that would otherwise carry oxygen. At low levels, carbon monoxide decreases the amount of oxygen supplied to the brain and other organs and muscles, slows body reactions and reflexes, and impairs judgment. It poses a serious threat to people with heart disease because of the fragile condition of the circulatory system and to fetuses because of the oxygen needs of the developing brain. At high levels, it can be fatal, as evidenced by numerous deaths caused by cars that are warmed up in closed garages or by exhaust gases leaking into the cars.
Smog also contains suspended particulate matter such as dust and soot emitted by vehicles and industrial facilities. Such particles irritate the eyes and the lungs since they may carry compounds such as acids and metals.
Acid Rain
Fossil fuels are mixtures of various chemicals, including small amounts of sulfur. The sulfur in the fuel reacts with oxygen to form sulfur dioxide (SO2), which is an air pollutant. The main source of SO2 is the electric power plants that burn high-sulfur coal. The Clean Air Act of 1970 has limited the SO2 emissions severely, which forced the plants to install SO2 scrubbers, to switch to low-sulfur coal, or to gasify the coal and recover the sulfur. Motor vehicles also contribute to SO2 emissions since gasoline and diesel fuel also contain small amounts of sulfur. Volcanic eruptions and hot springs also release sulfur oxides (the cause of the rotten egg smell).
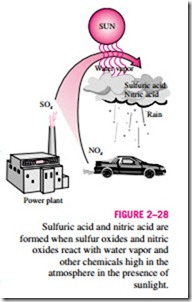
The sulfur oxides and nitric oxides react with water vapor and other chemicals high in the atmosphere in the presence of sunlight to form sulfuric and nitric acids (Fig. 2–28). The acids formed usually dissolve in the suspended water droplets in clouds or fog. These acid-laden droplets, which can be as acidic as lemon juice, are washed from the air on to the soil by rain or snow. This is known as acid rain. The soil is capable of neutralizing a certain amount of acid, but the amounts produced by the power plants using inexpen- sive high-sulfur coal has exceeded this capability, and as a result many lakes and rivers in industrial areas such as New York, Pennsylvania, and Michigan have become too acidic for fish to grow. Forests in those areas also experience a slow death due to absorbing the acids through their leaves, needles, and roots. Even marble structures deteriorate due to acid rain. The magnitude of the problem was not recognized until the early 1970s, and serious measures have been taken since then to reduce the sulfur dioxide emissions drastically by installing scrubbers in plants and by desulfurizing coal before combustion.
The Greenhouse Effect :Global Warming and Climate Change
You have probably noticed that when you leave your car under direct sunlight on a sunny day, the interior of the car gets much warmer than the air outside, and you may have wondered why the car acts like a heat trap. This is be- cause glass at thicknesses encountered in practice transmits over 90 percent of
radiation in the visible range and is practically opaque (nontransparent) to radiation in the longer wavelength infrared regions. Therefore, glass allows the solar radiation to enter freely but blocks the infrared radiation emitted by the interior surfaces. This causes a rise in the interior temperature as a result of the energy buildup in the car. This heating effect is known as the green- house effect, since it is utilized primarily in greenhouses.
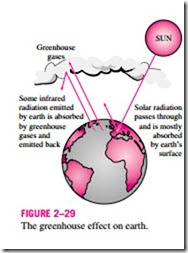
The greenhouse effect is also experienced on a larger scale on earth. The sur- face of the earth, which warms up during the day as a result of the absorption of solar energy, cools down at night by radiating part of its energy into deep space as infrared radiation. Carbon dioxide (CO2), water vapor, and trace amounts of some other gases such as methane and nitrogen oxides act like a blanket and keep the earth warm at night by blocking the heat radiated from the earth (Fig. 2–29). Therefore, they are called “greenhouse gases,” with CO2 being the primary component. Water vapor is usually taken out of this list since it comes down as rain or snow as part of the water cycle and human activities in producing water (such as the burning of fossil fuels) do not make much difference on its concentration in the atmosphere (which is mostly due to evaporation from rivers, lakes, oceans, etc.). CO2 is different, however, in that people’s activities do make a difference in CO2 concentration in the atmosphere.
radiation emitted by earth is absorbed by greenhouse gases and
warm (about 30°C warmer). However, excessive amounts of these gases disturb the delicate balance by trapping too much energy, which causes the average temperature of the earth to rise and the climate at some localities to change. These undesirable consequences of the greenhouse effect are referred to as global warming or global climate change.
The global climate change is due to the excessive use of fossil fuels such as coal, petroleum products, and natural gas in electric power generation, transportation, buildings, and manufacturing, and it has been a concern in recent decades. In 1995, a total of 6.5 billion tons of carbon was released to the atmosphere as CO2. The current concentration of CO2 in the atmosphere is about 360 ppm (or 0.36 percent). This is 20 percent higher than the level a century ago, and it is projected to increase to over 700 ppm by the year 2100. Under normal conditions, vegetation consumes CO2 and releases O2 during the photosynthesis process, and thus keeps the CO2 concentration in the atmosphere in check. A mature, growing tree consumes about 12 kg of CO2 a year and exhales enough oxygen to support a family of four. However, de- forestation and the huge increase in the CO2 production in recent decades disturbed this balance.
In a 1995 report, the world’s leading climate scientists concluded that the earth has already warmed about 0.5°C during the last century, and they estimate that the earth’s temperature will rise another 2°C by the year 2100. A rise of this magnitude is feared to cause severe changes in weather patterns with storms and heavy rains and flooding at some parts and drought in others, major floods due to the melting of ice at the poles, loss of wetlands and coastal areas due to rising sea levels, variations in water supply, changes in the ecosystem due to the inability of some animal and plant species to adjust to the changes, in- creases in epidemic diseases due to the warmer temperatures, and adverse side effects on human health and socioeconomic conditions in some areas.
The seriousness of these threats has moved the United Nations to establish a committee on climate change. A world summit in 1992 in Rio de Janeiro, Brazil, attracted world attention to the problem. The agreement prepared by
the committee in 1992 to control greenhouse gas emissions was signed by 162 nations. In the 1997 meeting in Kyoto (Japan), the world’s industrialized countries adopted the Kyoto protocol and committed to reduce their CO2 and other greenhouse gas emissions by 5 percent below the 1990 levels by 2008 to 2012. This can be done by increasing conservation efforts and improving con- version efficiencies, while meeting new energy demands by the use of renew- able energy (such as hydroelectric, solar, wind, and geothermal energy) rather than by fossil fuels.
The United States is the largest contributor of greenhouse gases, with over 5 tons of carbon emissions per person per year. A major source of greenhouse gas emissions is transportation. Each liter of gasoline burned by a vehicle produces about 2.5 kg of CO2 (or, each gallon of gasoline burned produces about 20 lbm of CO2). An average car in the United States is driven about 12,000 miles a year, and it consumes about 600 gallons of gasoline. Therefore, a car emits about 12,000 lbm of CO2 to the atmosphere a year, which is about four times the weight of a typical car (Fig. 2–30). This and other emissions can be reduced significantly by buying an energy-efficient car that burns less fuel over the same distance, and by driving sensibly. Saving fuel also saves money and the environment. For example, choosing a vehicle that gets 30 rather than 20 miles It is clear from these discussions that considerable amounts of pollutants are emitted as the chemical energy in fossil fuels is converted to thermal, mechanical, or electrical energy via combustion, and thus power plants, motor vehicles, and even stoves take the blame for air pollution. In contrast, no pollution is emitted as electricity is converted to thermal, chemical, or mechanical energy, and thus electric cars are often touted as “zero emission” vehicles and their widespread use is seen by some as the ultimate solution to the air pollution problem. It should be remembered, however, that the electricity used by the electric cars is generated somewhere else mostly by burning fuel and thus emit- ting pollution. Therefore, each time an electric car consumes 1 kWh of electricity, it bears the responsibility for the pollutions emitted as 1 kWh of electricity (plus the conversion and transmission losses) is generated else- where. The electric cars can be claimed to be zero emission vehicles only when the electricity they consume is generated by emission-free renewable resources such as hydroelectric, solar, wind, and geothermal energy (Fig. 2–31). There- fore, the use of renewable energy should be encouraged worldwide, with incentives, as necessary, to make the earth a better place to live in. The advancements in thermodynamics have contributed greatly in recent decades to improve conversion efficiencies (in some cases doubling them) and thus to re- duce pollution. As individuals, we can also help by practicing energy conservation measures and by making energy efficiency a high priority in our purchases.