REFRIGERATORS AND HEAT PUMPS
We all know from experience that heat flows in the direction of decreasing temperature, that is, from high-temperature mediums to low-temperature ones. This heat transfer process occurs in nature without requiring any de- vices. The reverse process, however, cannot occur by itself. The transfer of heat from a low-temperature medium to a high-temperature one requires special devices called refrigerators.
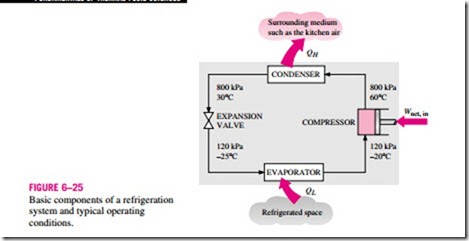
Refrigerators, like heat engines, are cyclic devices. The working fluid used in the refrigeration cycle is called a refrigerant. The most frequently used refrigeration cycle is the vapor-compression refrigeration cycle, which involves four main components: a compressor, a condenser, an expansion valve, and an evaporator, as shown in Fig. 6–25.
The refrigerant enters the compressor as a vapor and is compressed to the condenser pressure. It leaves the compressor at a relatively high temperature and cools down and condenses as it flows through the coils of the condenser by rejecting heat to the surrounding medium. It then enters a capillary tube where its pressure and temperature drop drastically due to the throttling effect. The low-temperature refrigerant then enters the evaporator, where it evaporates by absorbing heat from the refrigerated space. The cycle is completed as the refrigerant leaves the evaporator and reenters the compressor.
In a household refrigerator, the freezer compartment where heat is picked up by the refrigerant serves as the evaporator, and the coils behind the refrigerator where heat is dissipated to the kitchen air serve as the condenser.
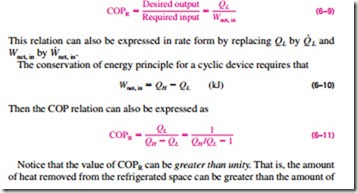
A refrigerator is shown schematically in Fig. 6–26. Here QL is the magnitude of the heat removed from the refrigerated space at temperature TL, QH is the magnitude of the heat rejected to the warm environment at temperature TH, and Wnet, in is the net work input to the refrigerator. As discussed before, QL and QH represent magnitudes and thus are positive quantities.
Coefficient of Performance
The efficiency of a refrigerator is expressed in terms of the coefficient of performance (COP), denoted by COPR. The objective of a refrigerator is to re- move heat (QL) from the refrigerated space. To accomplish this objective, it requires a work input of Wnet, in. Then the COP of a refrigerator can be ex- pressed as
work input. This is in contrast to the thermal efficiency, which can never be greater than 1. In fact, one reason for expressing the efficiency of a refrigera- tor by another term—the coefficient of performance—is the desire to avoid the oddity of having efficiencies greater than unity.
Heat Pumps
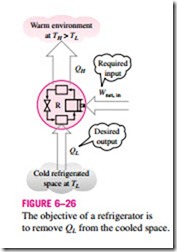
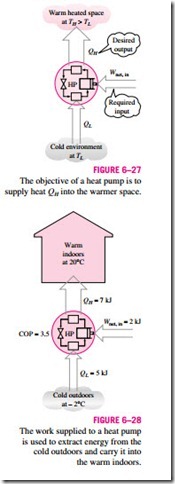
Another device that transfers heat from a low-temperature medium to a high temperature one is the heat pump, shown schematically in Fig. 6–27. Refrigerators and heat pumps operate on the same cycle but differ in their objectives. The objective of a refrigerator is to maintain the refrigerated space at a low temperature by removing heat from it. Discharging this heat to a higher-temperature medium is merely a necessary part of the operation, not the purpose. The objective of a heat pump, however, is to maintain a heated space at a high temperature. This is accomplished by absorbing heat from a low-temperature source, such as well water or cold outside air in winter, and supplying this heat to the high-temperature medium such as a house (Fig. 6–28).
An ordinary refrigerator that is placed in the window of a house with its door open to the cold outside air in winter will function as a heat pump since it will try to cool the outside by absorbing heat from it and rejecting this heat into the house through the coils behind it (Fig. 6–29).
The measure of performance of a heat pump is also expressed in terms of the coefficient of performance COPHP, defined as
for fixed values of QL and QH. This relation implies that the coefficient of performance of a heat pump is always greater than unity since COPR is a positive quantity. That is, a heat pump will function, at worst, as a resistance heater, supplying as much energy to the house as it consumes. In reality, however, part of QH is lost to the outside air through piping and other devices, and COPHP may drop below unity when the outside air temperature is too low. When this happens, the system usually switches to a resistance heating mode. Most heat pumps in operation today have a seasonally averaged COP of 2 to 3. Most existing heat pumps use the cold outside air as the heat source in winter, and they are referred to as air-source heat pumps. The COP of such heat pumps is about 3.0 at design conditions. Air-source heat pumps are not appropriate for cold climates since their efficiency drops considerably when temperatures are below the freezing point. In such cases, geothermal (also called ground-source) heat pumps that use the ground as the heat source can
be used. Geothermal heat pumps require the burial of pipes in the ground 1 to 2 m deep. Such heat pumps are more expensive to install, but they are also more efficient (up to 45 percent more efficient than air-source heat pumps). The COP of ground-source heat pumps is about 4.0.
Air conditioners are basically refrigerators whose refrigerated space is a room or a building instead of the food compartment. A window air- conditioning unit cools a room by absorbing heat from the room air and dis- charging it to the outside. The same air-conditioning unit can be used as a heat pump in winter by installing it backwards. In this mode, the unit will pick up heat from the cold outside and deliver it to the room. Air-conditioning systems that are equipped with proper controls and a reversing valve operate as air conditioners in summer and as heat pumps in winter.
The performance of refrigerators and air conditioners in the United States is often expressed in terms of the energy efficiency rating (EER), which is the amount of heat removed from the cooled space in Btu’s for 1 Wh (watt- hour) of electricity consumed. Considering that 1 kWh = 3412 Btu and thus 1 Wh = 3.412 Btu, a unit that removes 1 kWh of heat from the cooled space for each kWh of electricity it consumes (COP = 1) will have an EER of 3.412. Therefore, the relation between EER and COP is
Most air conditioners have an EER between 8 and 12 (a COP of 2.3 to 3.5). A high-efficiency heat pump recently manufactured by the Trane Com- pany using a reciprocating variable-speed compressor is reported to have a COP of 3.3 in the heating mode and an EER of 16.9 (COP of 5.0) in the air- conditioning mode. Variable-speed compressors and fans allow the unit to op- erate at maximum efficiency for varying heating/cooling needs and weather conditions as determined by a microprocessor. In the air-conditioning mode, for example, they operate at higher speeds on hot days and at lower speeds on cooler days, enhancing both efficiency and comfort.
The EER or COP of a refrigerator decreases with decreasing refrigeration temperature. Therefore, it is not economical to refrigerate to a lower tempera- ture than needed. The COPs of refrigerators are in the range of 2.6–3.0 for cut- ting and preparation rooms; 2.3–2.6 for meat, deli, dairy, and produce; 1.2–1.5 for frozen foods; and 1.0–1.2 for ice cream units. Note that the COP of freezers is about half of the COP of meat refrigerators, and thus it will cost twice as much to cool the meat products with refrigerated air that is cold enough to cool frozen foods. It is good energy conservation practice to use separate refrigeration systems to meet different refrigeration needs.
The Second Law of Thermodynamics: Clausius Statement
There are two classical statements of the second law—the Kelvin–Planck
statement, which is related to heat engines and discussed in the preceding section, and the Clausius statement, which is related to refrigerators or heat pumps. The Clausius statement is expressed as follows:
It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a lower-temperature body to a higher-temperature body.
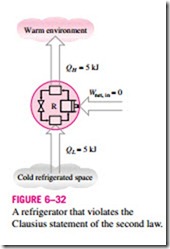
It is common knowledge that heat does not, of its own volition, flow from a cold medium to a warmer one. The Clausius statement does not imply that a cyclic device that transfers heat from a cold medium to a warmer one is im- possible to construct. In fact, this is precisely what a common household re- frigerator does. It simply states that a refrigerator will not operate unless its compressor is driven by an external power source, such as an electric motor (Fig. 6–32). This way, the net effect on the surroundings involves the con- sumption of some energy in the form of work, in addition to the transfer of heat from a colder body to a warmer one. That is, it leaves a trace in the sur- roundings. Therefore, a household refrigerator is in complete compliance with the Clausius statement of the second law.
Both the Kelvin–Planck and the Clausius statements of the second law are negative statements, and a negative statement cannot be proved. Like any other physical law, the second law of thermodynamics is based on experi- mental observations. To date, no experiment has been conducted that contra- dicts the second law, and this should be taken as sufficient evidence of its validity.
Equivalence of the Two Statements
The Kelvin–Planck and the Clausius statements are equivalent in their consequences, and either statement can be used as the expression of the second law of thermodynamics. Any device that violates the Kelvin–Planck statement also violates the Clausius statement, and vice versa. This can be demonstrated as follows.
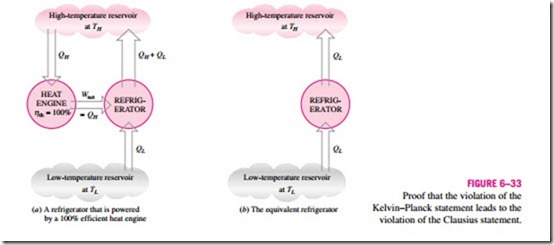
![]() Consider the heat-engine-refrigerator combination shown in Fig. 6–33a, op- erating between the same two reservoirs. The heat engine is assumed to have, in violation of the Kelvin–Planck statement, a thermal efficiency of 100 per- cent, and therefore it converts all the heat QH it receives to work W. This work is now supplied to a refrigerator that removes heat in the amount of QL from the low-temperature reservoir and rejects heat in the amount of QL + QH to the high-temperature reservoir. During this process, the high-temperature
Consider the heat-engine-refrigerator combination shown in Fig. 6–33a, op- erating between the same two reservoirs. The heat engine is assumed to have, in violation of the Kelvin–Planck statement, a thermal efficiency of 100 per- cent, and therefore it converts all the heat QH it receives to work W. This work is now supplied to a refrigerator that removes heat in the amount of QL from the low-temperature reservoir and rejects heat in the amount of QL + QH to the high-temperature reservoir. During this process, the high-temperature
reservoir receives a net amount of heat QL (the difference between QL + QH and QH). Thus, the combination of these two devices can be viewed as a refrigerator, as shown in Fig. 6–33b, that transfers heat in an amount of QL from a cooler body to a warmer one without requiring any input from outside. This is clearly a violation of the Clausius statement. Therefore, a violation of the Kelvin–Planck statement results in the violation of the Clausius statement. It can also be shown in a similar manner that a violation of the Clausius statement leads to the violation of the Kelvin–Planck statement. Therefore, the Clausius and the Kelvin–Planck statements are two equivalent expressions of the second law of thermodynamics.