■ DEW-POINT TEMPERATURE
If you live in a humid area, you are probably used to waking up most summer mornings and finding the grass wet. You know it did not rain the night before. So what happened? Well, the excess moisture in the air simply condensed on the cool surfaces, forming what we call dew. In summer, a considerable amount of water vaporizes during the day. As the temperature falls during the night, so does the “moisture capacity” of air, which is the maximum amount of moisture air can hold. (What happens to the relative humidity during this process?) After a while, the moisture capacity of air equals its moisture con- tent. At this point, air is saturated, and its relative humidity is 100 percent. Any further drop in temperature results in the condensation of some of the moisture, and this is the beginning of dew formation.
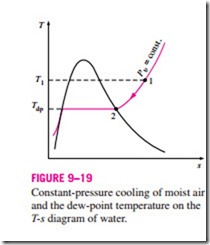
The dew-point temperature Tdp is defined as the temperature at which condensation begins when the air is cooled at constant pressure. In other words, Tdp is the saturation temperature of water corresponding to the vapor pressure:
This is also illustrated in Fig. 9–19. As the air cools at constant pressure, the vapor pressure Pυ remains constant. Therefore, the vapor in the air (state 1)
undergoes a constant-pressure cooling process until it strikes the saturated vapor line (state 2). The temperature at this point is Tdp, and if the temperature drops any further, some vapor condenses out. As a result, the amount of vapor in the air decreases, which results in a decrease in Pυ. The air remains saturated during the condensation process and thus follows a path of 100 percent relative humidity (the saturated vapor line). The ordinary temperature and the dew-point temperature of saturated air are identical.
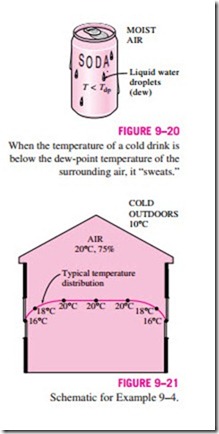
You have probably noticed that when you buy a cold canned drink from a vending machine on a hot and humid day, dew forms on the can. The formation of dew on the can indicates that the temperature of the drink is below the dew-point temperature of the surrounding air (Fig. 9–20).
The dew-point temperature of room air can be determined easily by cooling some water in a metal cup by adding small amounts of ice and stirring. The temperature of the outer surface of the cup when dew starts to form on the surface is the dew-point temperature of the air.