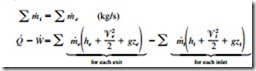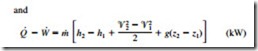SUMMARY
The first law of thermodynamics is essentially an expression of the conservation of energy principle, also called the energy balance. The general mass and energy balances for any system undergoing any process can be expressed as
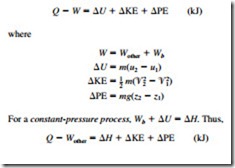
Taking heat transfer to the system and work done by the sys- tem to be positive quantities, the energy balance for a closed system can also be expressed as
Thermodynamic processes involving control volumes can be considered in two groups: steady-flow processes and unsteady- flow processes. During a steady-flow process, the fluid flows through the control volume steadily, experiencing no change with time at a fixed position. The mass and energy content of the control volume remain constant during a steady-flow process. Taking heat transfer to the system and work done by the system to be positive quantities, the conservation of mass and energy equations for steady-flow processes are ex- pressed as
where the subscript i stands for inlet and e for exit. These are the most general forms of the equations for steady-flow processes. For single-stream (one-inlet–one-exit) systems such as nozzles, diffusers, turbines, compressors, and pumps, they simplify to
In these relations, subscripts 1 and 2 denote the inlet and exit states, respectively.
Most unsteady-flow processes can be modeled as a uniform- flow process, which requires that the fluid flow at any inlet or exit is uniform and steady, and thus the fluid properties do not change with time or position over the cross section of an inlet or exit. If they do, they are averaged and treated as constants for the entire process. The energy balance for a uniform-flow system is expressed explicitly as
When the kinetic and potential energy changes associated with the control volume and fluid streams are negligible, the energy relation simplifies to
When solving thermodynamic problems, it is recommended that the general form of the energy balance �Esystem be used for all problems, and simplify it for the particular problem instead of using the specific relations given here for different processes.