■ REFRIGERATORS AND HEAT PUMPS
We all know from experience that heat flows in the direction of decreasing temperature, that is, from high-temperature regions to low-temperature ones. This heat transfer process occurs in nature without requiring any devices. The reverse process, however, cannot occur by itself. The transfer of heat from a low-temperature region to a high-temperature one requires special devices called refrigerators.
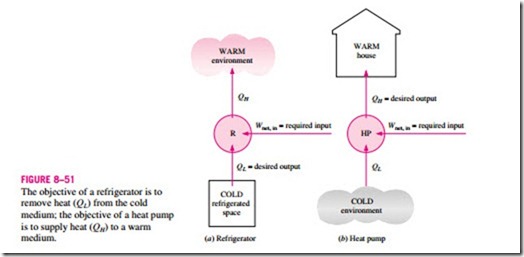
Refrigerators are cyclic devices, and the working fluids used in the refrigeration cycles are called refrigerants. A refrigerator is shown schematically in Fig. 8–51a. Here QL is the magnitude of the heat removed from the refrigerated space at temperature TL, QH is the magnitude of the heat rejected to the warm space at temperature TH, and Wnet, in is the net work input to the refrigerator. As discussed before, QL and QH represent magnitudes and thus are positive quantities.
Another device that transfers heat from a low-temperature medium to a high-temperature one is the heat pump. Refrigerators and heat pumps are essentially the same devices; they differ in their objectives only. The objective of a refrigerator is to maintain the refrigerated space at a low temperature by removing heat from it. Discharging this heat to a higher-temperature medium is merely a necessary part of the operation, not the purpose. The objective of
a heat pump, however, is to maintain a heated space at a high temperature. This is accomplished by absorbing heat from a low-temperature source, such as well water or cold outside air in winter, and supplying this heat to a warmer medium such as a house (Fig. 8–51b).
The performance of refrigerators and heat pumps is expressed in terms of the coefficient of performance (COP), which was defined as
for fixed values of QL and QH. This relation implies that COPHP > 1 since COPR is a positive quantity. That is, a heat pump will function, at worst, as a resistance heater, supplying as much energy to the house as it consumes. In reality, however, part of QH is lost to the outside air through piping and other devices, and COPHP may drop below unity when the outside air temperature is too low. When this happens, the system normally switches to the fuel (natural gas, propane, oil, etc.) or resistance-heating mode.
The cooling capacity of a refrigeration system—that is, the rate of heat removal from the refrigerated space—is often expressed in terms of tons of refrigeration. The capacity of a refrigeration system that can freeze 1 ton (2000 lbm) of liquid water at 0°C (32°F) into ice at 0°C in 24 h is said to be 1 ton. One ton of refrigeration is equivalent to 211 kJ/min or 200 Btu/min. The cooling load of a typical 200-m2 residence is in the 3-ton (10-kW) range.