gas laws
For all practical purposes, liquids used in hydraulic systems can be considered incompressible and insensitive to changes in temperature (provided the temperature remains within some quite broad limits). The gas in a pneumatic sys- tem is very sensitive to changes in pressure and temperature, and its behavior is determined by the gas laws described below.
In the following expressions it is important to note that pressures are given in absolute, not gauge, terms and temperatures are given in absolute degrees
Kelvin, not in degrees Celsius. If we discuss, say, a liter of air at atmospheric pressure and 20 °C being compressed to three atmospheres gauge pressure, its original pressure was one atmosphere, its original temperature was 293 K and its final pressure is four atmospheres absolute.
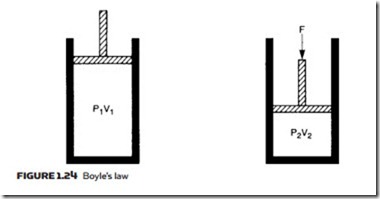
Pressure and volume are related by Boyle’s law. In Figure 1.24 we have a volume of gas V1 at pressure P1 (in absolute units, remember). This gas is com- pressed to volume V2, which will result in a rise of pressure to P2, where:
provided the temperature of the gas does not change during the compression. A reduction of pressure similarly leads to an increase in volume.
In practice, compression of a gas is always accompanied by a rise in tem- perature (as is commonly noticed when pumping up a bicycle tire) and a re- duction in pressure produces a temperature fall (the principle of refrigeration). For expression 1.17 to apply, the gas must be allowed to return to its original temperature.
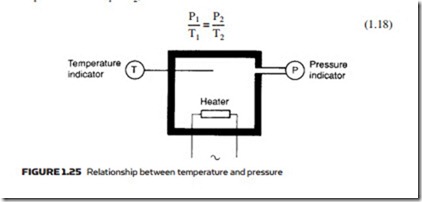
In Figure 1.25, on the other hand, the temperature of a fixed volume of gas is controlled by a heater. A rise in temperature from T1 to T2 results in an increase in pressure from P1 to P2, where:
Again it should be remembered pressure and temperature are in absolute terms. Although expression 1.18 gives the change in pressure resulting from a change in temperature, it also applies to changes in temperature resulting from a change in pressure provided no heat is lost from the system. In a pneumatic air compressor, the temperature of the outgoing compressed air is considerably elevated by the increase in pressure, resulting in the need for the compressor to be followed by an air cooler.
Expressions 1.17 and 1.18 are combined to give the general gas law:
where P1, V1, T1 are initial conditions and P2, V2, T2 are final conditions. As before, expression 1.19 assumes no heat is lost to, or gained from, the environment.