DRYING METHODS
There are three general methods of drying compressed air: chemical, adsorption, and refrigeration. In all cases, aftercooling and adequate condensation removal must be done ahead of this drying equipment. The initial and operating costs and the results obtained vary considerably.
These methods are primarily for water vapor removal. Removal of lubricating oil is secondary, although all drying systems will reduce its carryover. It should be under stood that complete elimination of lubricating oil, particularly in the vapor form, is very difficult and that when absolutely oil-free air is required, some form of nonlubricated compressor is the best guaranteed method.
Chemical Dryers
Chemical dryers are materials that combine with or absorb moisture from air when brought into close contact. There are two general types. One, using deliquescent material in the form of pellets or beads, is reputed to obtain a dew point, with 100•F inlet air to the dryer, of between 35 and so·F, depending on the specific type of deliquescent material. The material turns into a liquid as the water vapor is absorbed. This liquid must be drained off and the pellets or beads replaced periodically. Entering air above 9oo·p is not generally recommended.
The second type of chemical dryer utilizes an ethylene glycol liquid to absorb the moisture. Standard dew point reduction claimed is 400.F, but greater reductions are said to be possible with special equipment. The glycol is regenerated (i.e., dried) in a still using fuel gas or steam as a heating agent. The released moisture is vented to atmosphere. The regenerated glycol is recirculated by a pump through a water-cooled heat exchanger that lowers the glycol temperature before returning to the dryer vessel.
Adsorption
Adsorption is the property of certain extremely porous materials to hold vapors in the pores until the desiccant is either heated or exposed to a drier gas. The material is a solid at all times and operates alternately through drying and reactivation cycles with no change in composition. Adsorbing materials in principal use are activated alumina and silica gel. Molecular sieves are also used. Atmospheric dew points of -1,ooo·p are readily obtained using adsorption.
Reactivation or regeneration is usually obtained by diverting a portion of the already dried air through a reducing valve or orifice, reducing its pressure to atmospheric, and passing it through the wet desiccant bed. This air, with the moisture it has picked up from the saturated desiccant bed, is vented to atmosphere. The diverted air may vary from 7 to 17 percent of the main stream flow, depending upon the final dew point desired from the dryer. Heating the activating air prior to its passing through the desiccant bed, or heating the bed itself, is often done to improve the efficiency of the regeneration process. This requires less diverted air since each cubic foot of diverted air will carry much more moisture out of the system. Other modifications are also available to reduce or even eliminate the diverted air quantity.
Refrigeration
The use of refrigeration for drying compressed air is growing rapidly. It has been applied widely to small installations, to sections of larger plants, and even to entire manufacturing plant systems. Refrigerated air dryers have been applied to the air sys tem both before and after compression. In the before compression system, the air must be cooled to a lower temperature for a givenfinalline pressure dew point. This takes more refrigeration power for the same end result. Partially offsetting this is a saving in air compressor power per 1,000 cubic feet per minute (cfrn) of atmospheric air corn pressed due to the reduction in volume at the compressor inlet caused by the cooling and the removal of moisture. There is also a reduction in discharge temperature on single-stage compressors that may at times have some value. An atmospheric (inlet) dew point of 350’F is claimed.
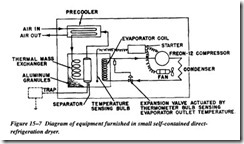
When air is refrigerated following compression, two systems have been used. Flow of air through directly refrigerated coils is used predominately in the smaller and moder ate-sized systems. These are generally standardized for cooling to 350’F, which is the dew point obtained at line pressure. Figure 15-7 diagrams the equipment furnished in a small self-contained direct-refrigeration dryer.
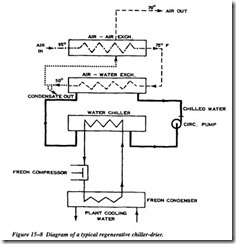
The larger systems chill water that is circulated through coils to cool the air. A dew point at line pressure of about 500’F is obtainable with this method. Figure 15-8 illus trates a typical system of a chiller-dryer unit. The designs shown are regenerative since the incoming air is partially cooled by the outgoing air stream. This reduces the size and first cost of the refrigeration compressor and exchanger. It also reduces
power cost and reheats the air returning to the line. Reheating of the air after it is dried has several advantages: the air volume is increased and less free air is required; chance of line condensation is still further reduced; and sweating of the cold pipe leaving the dryer is eliminated. Reheating dryers seldom need further reheating.
Combination Systems
The use of a combination dryer should be investigated when a very low dew point is necessary. Placing a refrigeration system ahead of an adsorption dryer will let the more economical refrigeration unit remove most of the vapor and reduce the load on the desiccant.