a Closer look at atoms
As previously stated, an atom is the smallest particle of an element. Atoms of different elements differ from each other. If there are over 100 known elements, then there are over 100 known atoms.
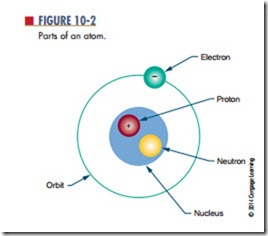
Every atom has a nucleus. The nucleus is located at the center of the atom. It contains positively charged particles called protons and uncharged particles called n e u t ro n s . Negatively charged particles called electrons orbit around the nucleus (Figure 10-2).
The number of protons in the nucleus of the atom is called the element’s atomic number. Atomic numbers distinguish one element from another.
Each element also has an atomic weight. The atomic weight is the mass of the atom and is deter- mined by the total number of protons and neutrons in the nucleus. Electrons do not contribute to the total mass of the atom; an electron’s mass is only 1/1845 that of a proton and is not significant enough to consider.
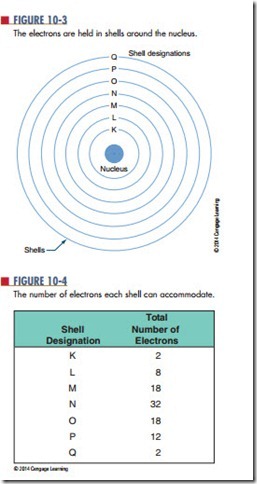
The electrons orbit in concentric circles about the nucleus. Each orbit is called a shell. These shells are filled in sequence; K is filled first, then L, M, N, and so on (Figure 10-3). The maximum number of electrons that each shell can accommodate is shown in Figure 10-4.
The outer shell is called the valence shell, and the number of electrons it contains is the valence. The farther the valence shell is from the nucleus, the less attraction the nucleus has on each valence electron. Thus the potential for the atom to gain or lose electrons increases if the valence shell is not full and is located far enough away from the nucleus. Conductivity of an atom depends on its valence band. The greater the number of electrons in the valence shell, the less it conducts. For example, an atom having seven electrons in the valence shell is less conductive than an atom having three electrons in the valence shell.
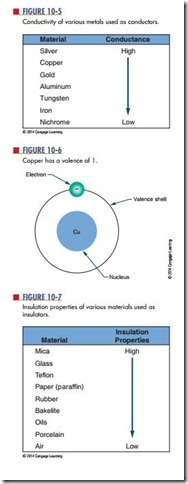
Electrons in the valence shell can gain energy. If these electrons gain enough energy from an external force, they can leave the atom and become free electrons, moving randomly from atom to atom. Materials that contain a large number of free electrons are called conductors. Figure 10-5 compares the conductivity of various metals used as conductors.
On the chart, silver, copper, and gold have a valence of 1 (Figure 10-6). However, silver is the best conductor because its free electron is more loosely bonded.
insulators, the opposite of conductors, prevent the flow of electricity. Insulators are stabilized by absorbing valence electrons from other atoms to fill their valence shells, thus eliminating free electrons. Materials classified as insulators are compared in Figure 10-7. Mica is the best insulator because it has the fewest free electrons in its valence shell. A perfect insulator has atoms with full valence shells. This means it cannot gain electrons.
Halfway between conductors and insulators are semiconductors. Semiconductors are neither good conductors nor good insulators but are important be- cause they can be altered to function as conductors or insulators. Silicon and germanium are two semiconductor materials.
An atom that has the same number of electrons and protons is identified as an electrically balanced atom. A balanced atom that receives one or more electrons is no longer balanced. It is said to be negatively charged and is called a negative ion. A balanced atom that loses one or more electrons is said to be positively charged and is called a positive ion. The process of gaining or losing electrons is called ionization. Ionization is significant in current flow.
Questions
1. What atomic particle has a positive charge and a large mass?
2. What atomic particle has no charge at all?
3. What atomic particle has a negative charge and a small mass?
4. What does the number of electrons in the outer- most shell determine?
What is the term for describing the gaining or losing of electrons?