Everything, whether natural or artificial, can be broken down into either an element or a compound. However, the smallest part of each of these is the atom.
The atom is made up of protons, neutrons, and electrons. The protons and neutrons group together to form the center of the atom, called the nucleus. The electrons orbit the nucleus in shells located at various distances from the nucleus.
When appropriate external force is applied to electrons in the outermost shell, they are knocked loose and become free electrons. The movement of free electrons is called current. The external force needed to create this current is called voltage. As it travels along its path, the current encounters some opposition, called resistance.
This chapter looks at how current, voltage, and resistance collectively form the fundamentals of electricity.
Matter, elements, and Compounds
Matter is anything that occupies space and has weight. It may be found in any one of three states: solid, liquid, or gas. Examples of matter include the air we breathe, the water we drink, the clothing we wear, and ourselves. Matter may be either an element or a compound.
An element is the basic building block of nature. It is a substance that cannot be reduced to a simpler substance by chemical means. There are now over 100 known elements (Appendix 1). Examples of elements are gold, silver, copper, and oxygen.
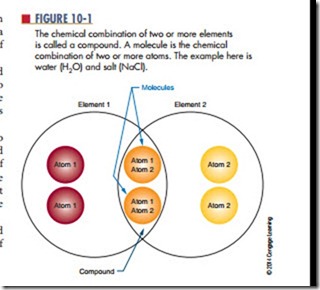
The chemical combination of two or more elements is called a compound (Figure 10-1). A compound can be separated by chemical, but not by physical means. Examples of compounds are water, which consists of hydrogen and oxygen, and salt, which consists of sodium and chlorine. The smallest part of the compound that still retains the properties of the compound is called a molecule. A molecule is the chemical combination of two or more atoms. An atom is the smallest particle of an element that retains the characteristic of the element. The physical combination of elements and compounds is called a mixture. Examples of mixtures include air, which is made up of oxygen, nitrogen, carbon dioxide, and other gases, and salt water, which consists of salt and water.
Questions
1. In what forms can matter be found?
2. What is a substance called that cannot be reduced to a simpler substance by chemical means?
3. What is the smallest possible particle that retains the characteristic of a compound?
4. What is the smallest possible particle that retains the characteristic of an element?
5. What is the combination of elements and compounds called?
Related posts:
Incoming search terms:
- basic electricity element and compound
- defination class 12 and uses and working and parts of circuit and its logic and mixture and combination and their role and ac aand dc motors triode diode electric circuits semiconductor capacitor resitor zener diode ammeter inductor coils voltmeter jockey
- matter element and compound of electricity
- matters element and compounds about electricity