■ DRY AND ATMOSPHERIC AIR
Air is a mixture of nitrogen, oxygen, and small amounts of some other gases. Air in the atmosphere normally contains some water vapor (or moisture) and is referred to as atmospheric air. By contrast, air that contains no water vapor is called dry air. It is often convenient to treat air as a mixture of water vapor and dry air since the composition of dry air remains relatively constant, but the amount of water vapor changes as a result of condensation and evaporation from oceans, lakes, rivers, showers, and even the human body. Although the amount of water vapor in the air is small, it plays a major role in human comfort. Therefore, it is an important consideration in air-conditioning applications.
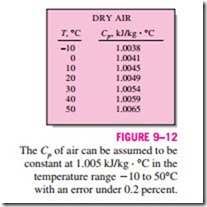
The temperature of air in air-conditioning applications ranges from about -10 to about 50°C. In this range, dry air can be treated as an ideal gas with a constant Cp value of 1.005 kJ/(kg · K) [0.240 Btu/(lbm · R)] with negligible error (under 0.2 percent), as illustrated in Fig. 9–12. Taking 0°C as the reference temperature, the enthalpy and enthalpy change of dry air can be determined from
where T is the air temperature in °C and LlT is the change in temperature. In air-conditioning processes we are concerned with the changes in enthalpy Llh, which is independent of the reference point selected.
It certainly would be very convenient to also treat the water vapor in the air as an ideal gas and you would probably be willing to sacrifice some accuracy for such convenience. Well, it turns out that we can have the convenience without much sacrifice. At 50°C, the saturation pressure of water is 12.3 kPa. At pressures below this value, water vapor can be treated as an ideal gas with negligible error (under 0.2 percent), even when it is a saturated vapor. There- fore, water vapor in air behaves as if it existed alone and obeys the ideal-gas relation Pυ = RT. Then the atmospheric air can be treated as an ideal-gas mixture whose pressure is the sum of the partial pressure of dry air* Pa and that of water vapor Pυ:
The partial pressure of water vapor is usually referred to as the vapor pressure. It is the pressure water vapor would exert if it existed alone at the temperature and volume of atmospheric air.
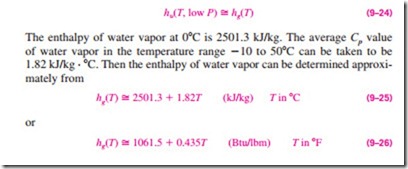
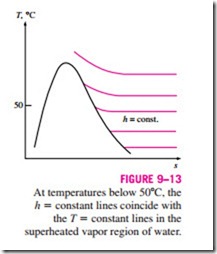
Since water vapor is an ideal gas, the enthalpy of water vapor is a function of temperature only, that is, h = h(T). This can also be observed from the T-s diagram of water given in Fig. A–9 and Fig. 9–13 where the constant-enthalpy lines coincide with constant-temperature lines at temperatures below 50°C. Therefore, the enthalpy of water vapor in air can be taken to be equal to the enthalpy of saturated vapor at the same temperature. That is,
in the temperature range -10 to 50°C (or 15 to 120°F), with negligible error, as shown in Fig. 9–14.