SPECIFIC AND RELATIVE HUMIDITY OF AIR
The amount of water vapor in the air can be specified in various ways. Probably the most logical way is to specify directly the mass of water vapor present in a unit mass of dry air. This is called absolute or specific humidity (also called humidity ratio) and is denoted by :
where P is the total pressure.
Consider 1 kg of dry air. By definition, dry air contains no water vapor, and thus its specific humidity is zero. Now let us add some water vapor to this dry air. The specific humidity will increase. As more vapor or moisture is added, the specific humidity will keep increasing until the air can hold no more moisture. At this point, the air is said to be saturated with moisture, and it is called saturated air. Any moisture introduced into saturated air will condense. The amount of water vapor in saturated air at a specified temperature and pressure can be determined from Eq. 9–29 by replacing Pυ by Pg, the saturation pressure of water at that temperature (Fig. 9–15).
The amount of moisture in the air has a definite effect on how comfortable we feel in an environment. However, the comfort level depends more on the amount of moisture the air holds (mυ) relative to the maximum amount of moisture the air can hold at the same temperature (mg). The ratio of these two quantities is called the relative humidity f (Fig. 9–16)
The relative humidity ranges from 0 for dry air to 1 for saturated air. Note that the amount of moisture air can hold depends on its temperature. Therefore, the relative humidity of air changes with temperature even when its specific humidity remains constant.
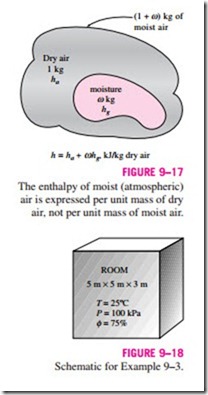
Atmospheric air is a mixture of dry air and water vapor, and thus the enthalpy of air is expressed in terms of the enthalpies of the dry air and the water vapor. In most practical applications, the amount of dry air in the air–water-vapor mixture remains constant, but the amount of water vapor changes. Therefore, the enthalpy of atmospheric air is expressed per unit mass of dry air instead of per unit mass of the air–water-vapor mixture.
The total enthalpy (an extensive property) of atmospheric air is the sum of the enthalpies of dry air and the water vapor:
Also note that the ordinary temperature of atmospheric air is frequently referred to as the dry-bulb temperature to differentiate it from other forms of temperatures that shall be discussed.