Heating Fundamentals
There is still considerable disagreement about the exact na ture of heat, but most authorities agree that it is a particular form of energy. Specifically , heat is a form of energy not associated with matter and in transit between its source and destination point. Furthermore , heat energy only exists as such between these two points. In other words , it only exists as heat energy while flowing between the source and destination.
So far this description of heat energy has been practically identical to that of work energy, the other form of energy in tran sit not associated with matter. The distinguishing difference be tween the two is that heat energy is energy in transit as a result of temperature differences between its source and destination point , whereas work energy in transit is due to other, non-tem perature factors.
BRITISH THERMAL UNIT
Heat energy is measured by the British thermal unit (Btu). Each British thermal unit is regarded as equivalent to one unit of heat (heat energy).
Since 1929 British thermal units have been defined on the ba sis of one Btu being equal to 251.996 IT (International Steam Table) calories , or 778.26 ft.-lb. of mechanical energy units (work). Taking into consideration that one IT calorie equals Ys 60 of a watt-hour, one Btu is then equivalent to about % watt hour.
Prior to its redefinition in 1929, one Btu was defined as the amount of heat necessary to raise 1 lb. of water 1°F. Because of the difficulty in determining the exact value of a Btu, it was later redefined in terms of the more fundamental electrical unit.
RELATIONSHIP BETWEEN HEAT AND WORK
Energy is the ability to do work or move agamst a resistance. Conversely, work is the overcoming of resistance through a certain distance by the expenditure of energy.
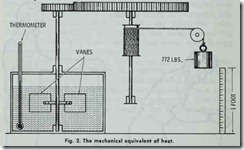
Work is measured by a standard unit called the foot pound. The latter may be defined as the amount of work done in raising one pound the distance of one foot, or in overcoming a pressure of one pound through a distance of one foot (Fig. 1) .
The relationship between work and heat is referred to as the mechanical equivalent of heat. That is to say, one unit of heat is equal to 778.26 foot-pounds of work.
This relationship (i.e. the mechanical equivalent of heat) was first established by experiments conducted in the nineteenth cen tury. In 1843 Dr. James Prescott Joule (1818-1889) of Man chester, England determined by numerous experiments that when 772 foot-pounds of energy had been expended on one pound of water, the temperature of the latter had risen one degree, and the relationship between heat and mechanical work was found (Fig. 2). The value 772 foot-pounds is known as Joule’s equivalent. More recent experiments give higher figures, and the value 778
( 1 Btu = 778.26 ft.-lbs. See BRITISH THERMAL UNITS in this chapter).
HEAT TRANSFER
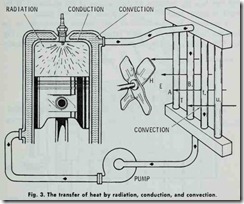
When bodies of unequal temperatures are placed near each other, heat leaves the hotter body and is absorbed by the colder one until the temperature of each is equal. The rate by which the heat is absorbed by the colder body is proportional to the differ ence of temperature between the two bodies-the greater the dif ference of temperature the greater the rate of flow of the heat.
Heat is transferred from one body to another at lower temper ature by any one of the following means (Fig. 3):
1. Radiation,
2. Conduction,
3. Convection.
Radiation, insofar as heat loss is concerned, refers to the throwing out of heat in rays. The heat rays proceed in straight lines and the intensity of the heat radiated from any one source becomes less as the distance from the source increases.
The amount of heat loss through radiation will depend upon the temperature of the floor, ceiling, and walls in the room or building. The colder these surfaces are, the faster and greater will be the heat loss from a human body standing within the enclosure. On the other hand, if the wall, ceiling, and floor surfaces are warmer than the human body within the enclosure they form, heat will be radiated from these surfaces to the body. In these situations a person may complain that the room is too hot.
Knowledge of the (mean radiant) temperature of the surfaces of an enclosure is important when dealing with heat loss by radi ation. The mean radiant temperature (MRT) is the weighted average temperature of these surfaces (i.e. the floor, ceiling, and walls). The significance of the mean radiant temperature is de termined when compared with the clothed body of an adult (goo F. or 26.7 °C.). If the MRT is below goo F. the human body will lose heat by radiation to the surfaces of the enclosure. If the MRT is higher than go oF. the opposite effect will occur.
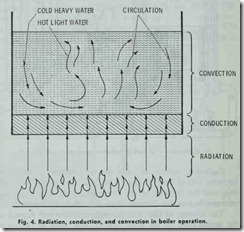
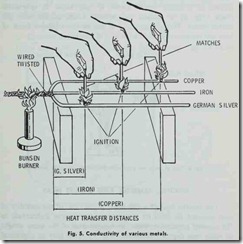
Conduction is the transfer of heat through substances, as for instance , boiler plate to another substance in contact with it (Fig. 4). Conductivity may be defined as the relative value of a material as compared with a standard, in affording a passage through itself or over its surface for heat. A poor conductor is usually re ferred to as a nonconductor or insulator. Copper is an example of a good conductor. Fig. 5 illustrates the comparative heat con ductivity rates of three frequently used metals. The various ma terials used to insulate buildings are poor conductors. It.should be pointed out that any substance, which is a good conductor of electricity, is als a good conductor of heat.
Convection is the transfer of heat by the motion of the heated matter itself. Because motion is a required aspect of the defini tion of convection, it can therefore take place only in liquids and gases.
Fig. 4 illustrates how radiation, conduction, and convection are often interrelated. Heat from the burning fuel passes to the metal of the heating surface by radiation. through the metal by conduction, and is transferred to the water by convection (i.e. circulation). Circulation is caused by a variation in the weight of the water due to temperature differences. That is, the water next to the heating surface receives heat and expands (becomes lighter) and immediately rises due to displacement by the colder and heavier water above.
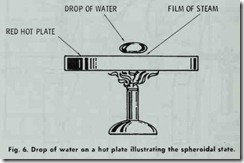
Proper circulation is very important because its absence will cause a liquid, such as water, to reach the spheroidal state. This in turn causes the metal of the boiler to become dangerously overheated. A liquid that has reached the spheroidal state is easy to recognize by its appearance. When liquid is dropped upon the surface of a highly heated metal, it rolls about in spheroidal drops (Fig. 6) or masses, and without actual contact with the heated metal. This phenomenon is due to the repulsive force of heat and the intervention of a cushion of steam.
SPECIFIC, SENSIBLE AND LATENT HEAT
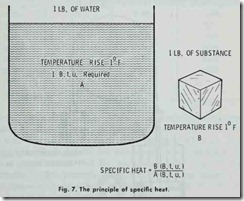
The specific heat of a substance is the ratio of the quantity of heat required to raise its temperature one degree Fahrenheit to the amount required to raise the temperature of the same weight of water one degree Fahrenheit (Fig. 7). This may be expressed in the following formula:
The standard used is water at approximately 62°F. to 63°F. which receives a rating of 1.00 on the specific heat scale. Simply stated, specific heat represents the Btu’s required to raise the temperature of 1 lb. of a substance 1°F.
Sensible heat is that part of heat which provides temperature change and which can be measured by a thermometer. It is referred to as such because it can be “sensed” by instruments or the touch.
Latent heat is that quantity of heat which disappears or becomes concealed in a body while producing some change in it other than a rise of temperature. Changing a liquid to a gas, or a gas to a liquid are both activities involving latent heat. The two types of latent heat are:
I. Internal latent heat.
2. External latent heat.
These are explained in detail in a later section (see STEAM in this chapter).
HEAT CONVEYING MEDIUMS
As was mentioned in the previous chapter, there are a variety of ways to classify heating systems. One method is based on the medium which conveys the heat from its source to the point being heated. When the majority of heating systems in use today are examined closely. it can be seen that there are only four basic heat conveying mediums involved:
I. Air,
2. Steam,
3. Water,
4. Electricity.
Air
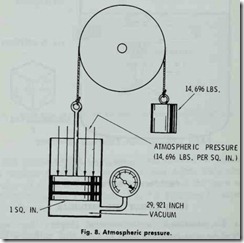
Air is a gas cons1stmg of a mechanical mixture of 23.2% oxygen (by weight), 75.5% nitrogen, and 1.3% argon with small amounts of other gases. It functions as the heat conveying medium for hot-air heating systems.Atmospheric pressure may be defined as the force exerted by the weight of the atmosphere in every point with which it is in contact (Fig. 8), and is measured in inches of mercury or the corresponding pressure in lbs. per square inch.
The pressure of the atmosphere is approximately 14.7 lbs. per square inch at sea level. The standard atmosphere is 29.921 inches of mercury at 14.696 lbs. per square inch. The term “inches of mercury” refers to the height to which the column of mercury in the barometer will remain suspended to balance the pressure due to the weight of the atmosphere.
Atmospheric pressure will vary due to elevation by decreas ing approximately one half pound for every 1000 feet ascent above sea level. Atmospheric pressure in lbs. per square inch is obtained from the barometer reading by multiplying the baro meter reading in inches by 0.49116. Examples are given in Table 1.
Table 1. Pressure of the atmosphere per square inch for various readings of the barometer.
|
Barometer (ins. of mercury) |
Pressure per sq. ins., lbs. |
Barometer (ins. of mercury) |
Pressure per sq. ins., lbs. |
|
28.00 |
13.75 |
29.921 |
14.696 |
|
28.25 |
13.88 |
30.00 |
14.74 |
|
28.50 |
14.00 |
30.25 |
14.86 |
|
28.75 |
14.12 |
30.50 |
14.98 |
|
29.00 |
14.24 |
30.75 |
15.10 |
|
29.25 |
14.37 |
31.00 |
15.23 |
|
29.50 |
14.49 |
||
|
29.75 |
14.61 |
Gauge pressure is pressure whose scale starts at atmospheric pressure. Absolute pressure, on the other hand, is pressure mea sured from true zero or the point of no pressure. When the hand of a steam gauge is at zero, the absolute pressure existing in the boiler is approximately 14.7 lbs. per square inch. Thus, by way of example, 5 lbs. pressure measured by a steam gauge (i.e. gauge pressure) is equal to 5 lbs. plus 14.7 lbs. or 19.7 lbs. per square inch of absolute pressure.
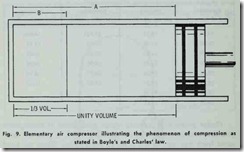
When air is compressed, both its pressure and temperature are changed in accordance with Boyle’s and Charles’ laws. Ac cording to Robert Boyle ( 1627-1691 ) , the English philosopher and founder of modern chemistry, the absolute pressure of a gas at constant temperature varies inversely as its volume. Jacques Charles (1746-1823), on the other hand, established that the volume of a gas is proportional to its absolute temperature when the volume is kept at constant pressure.
If the cylinder in Fig. 9 is filled with air at atmospheric pressure ( 14.7 lbs. per square inch absolute). represented by volume A, and the piston B moved to reduce the volume to say 1;3 A, as represented by B, then according to Boyle’s law, the
pressure will be trebled (14.7 X 3 = 44.1 lbs absolute or 44.1 – 14.7 = 29.4 gauge pressure). According to Charles’law, a pressure gauge on the cylinder would at this point indicate a higher pressure than 29.4 gauge pressure because of the in crease in temperature produced by compressing the air. This is called adiabatic compression if no heat is lost, or received externally.
Steam
Those who design, install, or have charge of steam heating plants certainly should have some knowledge of steam, its forma tion and behavior under various conditions.
Steam is a colorless expansive and invisible gas resulting from the vaporization of water. The white cloud associated with steam is a fog of minute liquid particles formed by condensation. That is to say, finely divided condensation. This white cloud is caused by the exposure of the steam to a temperature lower than that corresponding to its pressure.
If the inside of a steam heating main were visible, it would be filled part way with a white cloud and in traversing the main the little particles combine, forming drops of condensation too heavy to remain in suspension and accordingly drop to the bottom
of the main and drain off as condensation. This condensation flows into a drop leg of the system and finally back into the boiler, together with additional condensation draining from the radiators.
Although the word “steam” should only be applied to the saturated gas, the five following classes of steam are recognized:
1. Saturated steam,
2. Dry steam,
3. Wet steam,
4. Superheated steam,
5. Highly superheated or gaseous steam.
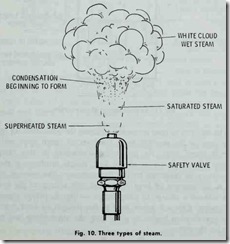
Three of these classes of steam (wet, saturated , and super heated) are shown in the illustration of a safety valve blowing in Fig. 10. It should be pointed out that neither saturated steam nor superheated steam can be seen by the naked eye.
Saturated steam may be defined as steam of a temperature due to its pressure. Steam containing intermingled moisture , mist , or spray, is referred to as wet steam. Dry steam, on the other hand , is steam containing no moisture. It may be either saturated or superheated. Finally , superheated steam is steam having a temperature higher than that corresponding to its pressure.
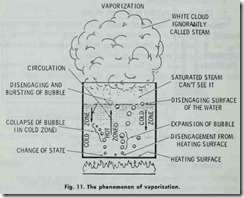
The variou s changes which take place in the making of steam are known as vaporization, and are shown in Fig. 11. For the sake of illustration , only one bubble is shown in each receptacle. In actuality there is a continuous procession upward of a great multiplic ity of bubbles.
The amount of heat necessary to cause the generation of steam is the sum of the sensible heat , the internal latent heat, and the extern al latent h eat. As mentioned elsewhere in this chapter, sensible heat is that part of the heat which produces a rise in temperatur e as indicated by the thermometer. The internal latent heat is the amount of heat that water will absorb at the boiling point without a change in temperature -that is, before vaporiza tion begins. External latent heat is the amount of heat required when vaporization begins to push back the atmosphere and make room for the steam.
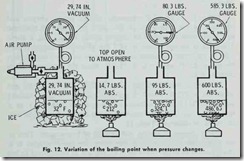
Another important factor to consider when dealing with steam is the boiling point of liquids. By definition, the boiling point is the temperature at which a liquid begins to boil (Fig. 12), and it depends upon both the pressure and nature of the liquid. For instance, water boils at 212°F. and ether at 9°F. under atmospheric pressure of 14.7 lbs. per square inch.
The relationship between boiling point and pressure is such that there is a definite temperature or boiling point corresponding to each value of pressure. When vaporization occurs in a closed vessel and there is a temperature rise, the pressure will rise until the equilibrium between temperature and pressure is re-estab lished.
One’s knowledge of the fundamentals of steam heating should also include an understanding of the role that condensation plays. By definition, condensation is the change of a substance from the gaseous to the liquid (or condensate) form. This change is caused by a reduction in temperature of the steam below that corres ponding to its pressure.
The condensation of steam can cause certain problems for steam heating systems unless they are designed to allow for it. The water from which the steam was originally formed contained,
mechanically mixed with it, Yzo or 5 percent of its volume of air (at atmospheric pressure). This air is liberated during vaporization and does not re-combine with the condensation. As a result, trouble is experienced in heating systems when attempting to get the air out and keep it out. Suitable air valves are necessary to correct the problem.
Water
Water is a chemical compound of two gases, oxygen and hydrogen in the proportion of two parts by weight of hydrogen to sixteen parts by weight of oxygen having mixed with it about 5 percent of air by volume at 14.7 lbs. absolute pressure. It may exist as ice, water, or steam due to changes in temperature (water freezes at 32°F. and boils at 212°F. when the barometer reads 29.921 inches).
One cubic foot of water weighs 62.41 lbs. at 32°F. and 59.82lbs. at 212°F. One U.S. gallon of water (231 cubic inches) weighs 8.33111 lbs. (ordinarily expressed as 8lh lbs.) at a temperature of 62°F. At any other temperature the weight will, of course, be different (Table 2).
Water changes in weight with change of temperature. That is, the higher the temperature of the water, the less it weighs. It is this property of water that causes circulation in boilers and in hot water heating systems. The change in weight is due to expansion and a reduction in water volume. As the temperature rises, the water expands and results in a unit volume of water containing less water at higher temperature than lower temperature.
Fill a vessel with cold water and heat it to the boiling point. Note that boiling causes it to overflow due to expansion. Now let the water cool. You will note that when the water is cold the vessel will not be as full because the water will have contracted.
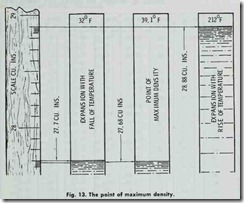
The point of maximum density of water is 39.1 °F. The most remarkable characteristic of water is its expansion below and above its point of maximum density. Imagine one pound of water at 39.1°F. placed in a cylinder having a cross sectional area of 1 square inch (Fig. 13). The water having a volume of 27.68 cubic inches will fill the cylinder to a height of 27.68 inches. If the water is cooled it will expand, and at say 32°F. (the freezing point), will rise in the tube to a height of 27.7 inches before
Table 2. Weight of water per cubic foot
|
Temp., deg. F. |
Lb. per cu. ft. |
Temp., deg. F. |
Lb. per cu. ft. |
Temp., deg. F. |
Lb. per cu. ft. |
Temp., deg. F. |
Lb. per cu. ft. |
|
32 |
62.41 |
62 |
62.35 |
91 |
62.10 |
121 |
61.69 |
|
33 |
62.41 |
63 |
62.34 |
92 |
62.08 |
122 |
61.68 |
|
34 |
62.42 |
64 |
62.34 |
93 |
62.07 |
123 |
61.66 |
|
35 |
62.42 |
65 |
62.33 |
94 |
62.06 |
124 |
61.64 |
|
36 |
62.42 |
66 |
62.32 |
95 |
62.05 |
125 |
61.63 |
|
37 |
62.42 |
67 |
62.32 |
96 |
62.04 |
126 |
61.61 |
|
38 |
62.42 |
68 |
62.31 |
97 |
62.02 |
127 |
61.60 |
|
39 |
62.42 |
69 |
62.30 |
98 |
62.01 |
128 |
61.58 |
|
40 |
62.42 |
70 |
62.30 |
99 |
62.00 |
129 |
61.56 |
|
41 |
62.42 |
71 |
62.29 |
100 |
61.99 |
130 |
61.55 |
|
42 |
62.42 |
72 |
62.28 |
101 |
61.98 |
131 |
61.53 |
|
43 |
62.42 |
73 |
62.27 |
102 |
61.96 |
132 |
61.51 |
|
44 |
62.42 |
74 |
62.26 |
103 |
61.95 |
133 |
61.50 |
|
45 |
62.42 |
75 |
62.25 |
104 |
61.94 |
134 |
61.48 |
|
46 |
62.41 |
76 |
62.25 |
105 |
61.93 |
135 |
61.46 |
|
47 |
62.41 |
I 77 |
62.24 |
106 |
61.91 |
136 |
61.44 |
|
48 |
62.41 |
78 |
62.23 |
107 |
61.90 |
137 |
61.43 |
|
49 |
62.41 |
79 |
62.22 |
108 |
61.89 |
138 |
61.41 |
|
50 |
62.40 |
80 |
62.21 |
109 |
61.87 |
139 |
61.39 |
|
51 |
62.40 |
81 |
62.20 |
110 |
61.86 |
140 |
61.37 |
|
52 |
62.40 |
82 |
62.19 |
111 |
61.84 |
141 |
61.36 |
|
53 |
62.39 |
83 |
62.18 |
112 |
61.83 |
142 |
61.34 |
|
54 |
62.39 |
84 |
62.17 |
113 |
61.81 |
143 |
61.32 |
|
55 |
62.38 |
85 |
62.16 |
114 |
61.80 |
144 |
61.30 |
|
56 |
62.38 |
86 |
62.15 |
115 |
61.78 |
145 |
61.28 |
|
57 |
62.38 |
87 |
62.14 |
116 |
61.77 |
146 |
61.26 |
|
58 |
62.37 |
88 |
61.13 |
117 |
61.75 |
147 |
61.25 |
|
59 |
62.37 |
89 |
62.12 |
118 |
61.74 |
148 |
61.23 |
|
60 |
62.36 |
90 |
62.11 |
119 |
61.72 |
149 |
61.21 |
|
61 |
62.35 |
120 |
61.71 |
freezing. If the water is heated, it will also expand and rise in the tube, and at the boiling point (for atmospheric pressure 212°F) will occupy the tube to a height of 28.88 cubic inches.
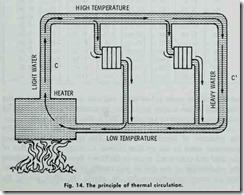
The elementary hot water heating system in Fig. 14 illustrates the principle of thermal circulation. The weight of the hot and expanded water in the up flow column C, being less than that of the cold and contracted water in the down flow Column C’ upsets the equilibrium of the system and results in a continuous circula tion of water as indicated by the arrows. In other words, the at different temperatures.
|
Temp., deg. F. |
Lb. per cu. ft. |
Temp., deg. F. |
Lb. per cu. ft. |
Temp., deg. F. |
Lb. per cu. ft. |
Temp., deg . F. |
Lb. per cu. ft. |
|
150 |
61.19 |
180 |
60.57 |
208 |
59.92 |
440 |
51.87 |
|
151 |
61.17 |
181 |
60.55 |
209 |
59.90 |
450 |
51.28 |
|
152 |
61.15 |
182 |
60.53 |
210 |
59.87 |
460 |
51.02 |
|
153 |
61.13 |
183 |
60.51 |
211 |
59.85 |
470 |
50.51 |
|
154 |
61.1 1 |
184 |
60.49 |
212 |
59.82 |
480 |
50.00 |
|
155 |
61.09 |
185 |
60.46 |
214 |
59.81 |
490 |
49.50 |
|
156 |
61.07 |
186 |
60.44 |
216 |
59.77 |
500 |
48.78 |
|
157 |
61.05 |
187 |
60.42 |
218 |
59.70 |
510 |
48.31 |
|
158 |
61.03 |
188 |
60.40 |
220 |
59.67 |
520 |
47.62 |
|
159 |
61.01 |
189 |
60.37 |
230 |
59.42 |
530 |
46.95 |
|
160 |
60.99 |
190 |
60.35 |
240 |
59.17 |
540 |
46.30 |
|
161 |
60.97 |
191 |
60.33 |
250 |
58.89 |
550 |
45.66 |
|
162 |
60.95 |
192 |
60.30 |
260 |
58.62 |
560 |
44.84 |
|
163 |
60.93 |
193 |
60.28 |
270 |
58.34 |
570 |
44.05 |
|
164 |
60.91 |
194 |
60.26 |
280 |
58.04 |
580 |
43.29 |
|
165 |
60.89 |
195 |
60.23 |
290 |
57.74 |
590 |
42.37 |
|
166 |
60.87 |
196 |
60.21 |
300 |
57.41 |
600 |
41.49 |
|
167 |
60.85 |
197 |
60.19 |
310 |
57.08 |
610 |
40.49 |
|
168 |
60.83 |
198 |
60.16 |
320 |
56.75 |
620 |
39.37 |
|
169 |
60.81 |
199 |
60.14 |
330 |
56.40 |
630 |
38.31 |
|
170 |
60.79 |
200 |
60.11 |
340 |
56.02 |
640 |
37.17 |
|
171 |
60.77 |
201 |
60.09 |
350 |
55.65 |
650 |
35.97 |
|
172 |
60.75 |
202 |
60.07 |
360 |
55.25 |
660 |
34.48 |
|
173 |
60.73 |
203 |
60.04 |
370 |
54.85 |
670 |
32.89 |
|
174 |
60.71 |
204 |
60.02 |
380 |
54.47 |
680 |
31.06 |
|
175 |
60.68 |
205 |
59.99 |
390 |
54.05 |
690 |
28.82 |
|
176 |
60.66 |
206 |
59.97 |
400 |
53.62 |
700 |
25.38 |
|
177 |
60.64 |
207 |
59.95 |
410 |
53.19 |
706.1 |
19.16 |
|
178 |
60.62 |
……… |
………….. |
420 |
52.74 |
………… |
…………… |
|
179 |
60.00 |
430 |
52.33 |
heavy low temperature water sinks to the lowest point in the boiler (or system) and displaces the light high temperature water , thus causing continuous circulation as long as there is a tempera ture difference in different parts of the boiler (or system). This is referred to as thermal circulation.
Electricity
Electricity differs from air, steam, or water in that it does not actually convey heat from one point to another; therefore, in eluding it in a list of heat conveying mediums can be misleading at first glance.
Electricity can best be defined as a quantity of electrons either in motion or in a state of rest. When these electrons are at rest, they are referred to as being static (hence the term “static elec tricity”). Electrons in motion move from one atom to another creating an electrical current, and thereby a medium for convey ing energy from one point to another. Many different types of devices have been created to change the energy conveyed by an electric current into heat, light, and other forms of energy. Electric-fired furnaces and boilers are examples of these devices used to produce heat.