Reaction Rates and the Chemical Constant
We consider a simple reaction of the type
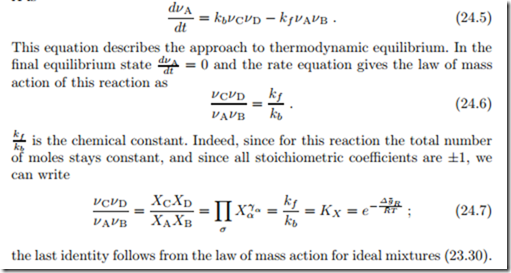
Accordingly, the rates of change of the mole densities να = nα/V of the involved species must be related as
We consider the rate of change of molecules of species A. Molecules of type A are produced in backward reactions at rate rb and are consumed in forward reactions at rate rf . The rate of change is just the difference between backward and forward reactions,
Each rate, rb or rf , is proportional to the probability to find the reaction partners at the same location, and to the probability that a reaction will actually take place when the partners meet. An intuitive choice for the rates is
where νCνD and νAνB are measures for the probability to find the reaction partners C − D or A − B, and kb, kf measure the reaction probability (and include scaling factors for the probability). The above are the simplest meaningful choices for the reaction rates; realistic reactions will have more complicated relations between reaction rates and mole densities.
According to the model, the rate of change of the mole density of species A is