Approaching Chemical Equilibrium
So far, we have considered chemical equilibrium, which stands at the end of a reactive process, but not the details of reaching the equilibrium. In the discussion of the ammonia synthesis, we have mentioned the need for catalysts, which facilitate the reactions, and are required to reach the equilibrium state in reasonable time. In the present chapter, we shall discuss reaction rates, and the activation of reactions. Activation losses are an important cause of losses in fuel cells, and thus we will come back to this topic in the discussion of thermodynamics of fuel cells.
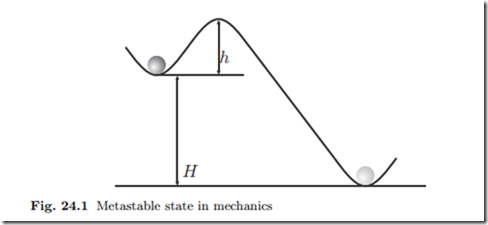
To introduce the problem, we consider a mechanical analogy, see Fig. 24.1. The stable state for the ball is the minimum at the bottom, but it is trapped in a metastable state at a local minimum at height H. In order to reach the absolute minimum, the ball has to overcome the well of height h, which requires energy.
For chemical reactions to occur, the reactant molecules must be split into parts that then can take part in forming the products. This requires energy, which can come, e.g., from collisional impact. As an example we consider the formation of water from hydrogen and oxygen. The reactants are gases, and even at high pressures, most collisions involve only two particles, while three- particle collisions are very rare. In the following list X denotes a collision partner that provides or removes energy to or from the collisions, but is not involved in the reaction itself. The reactions that happen in the formation of water are
From the statement that two-particle collisions are far more frequent than three-particle collisions one might conclude that the first two reactions will mainly happen in the forward direction (high energy impact of X on H2 or O2 splits these), while the last reaction will mainly happen in the backward direction (splitting of water by impact of X). The rate at which reactions take place will be proportional to the probability for a collision and to the probability that a reaction actually occurs when a collision takes place.