CARBON DIOXIDE REMOVAL
Limiting or completely removing carbon dioxide from the flue gases of a coal- fired power plant is likely to become necessary on new power plants after 2020. Even before that, increasingly onerous penalties for emitting the gas may make it necessary for power plant operators to find ways of curbing their carbon emissions (carbon emissions is often used as shorthand for carbon dioxide emis- sions). The technology to capture carbon dioxide already exists. It has not been deployed on a large coal-fired power plant but demonstration projects are being planned. However, this is only part of the problem. Carbon dioxide, once captured, must be stored (sequestered) in a way that prevents it ever entering the atmosphere. Otherwise the capture process will have been a waste of time. Therefore, carbon sequestering techniques must be perfected alongside carbon capture if the ultimate goal of carbon-emission-free coal combustion is ever to be realized.
For carbon capture there are three main approaches available. The first, often called post-combustion capture, involves installing a plant similar to an FGD scrubber to the exhaust of the power plant. Reagents capable of absorbing carbon dioxide are already available and this technology is likely to be one of the simplest and possibly the most economical to deploy. An alternative to this, called pre-combustion capture, involves pretreating coal to remove the carbon before combustion. This is achieved using a modified version of coal gasification that, when carried out fully, leaves a fuel gas composed primarily of hydro- gen. The gasification process also requires some form of carbon dioxide capture technology, which may be via a scrubber system too. The third way of tackling carbon dioxide emissions attempts to sidestep the difficult problem of separating carbon dioxide from the flue gases, which are mainly composed of nitrogen after combustion is over. Instead, oxygen is separated from air first, and then coal is burned in virtually pure oxygen. The scheme, called oxy-fuel combustion, leads to an exhaust gas stream composed primarily of carbon dioxide, mixed with some water vapor and excess oxygen, from which it is much easier to isolate the carbon dioxide than when the latter is mixed with nitrogen.
There is a further method of reducing, though not eliminating, carbon dioxide emissions in a coal-fired plant: by replacing some of the coal with a biofuel. Biofuel cofiring, as this technique is known, cannot be used to eliminate all car- bon emissions. For that to be possible the plant would have to burn biofuel alone, so it would no longer be a coal-fired plant. However, it can improve a coal plant’s environmental performance.
Post-combustion Capture
Biomass as a power plant fuel is generally considered to be carbon dioxide neutral. Combustion of biomass generates carbon dioxide in the same way as the combustion of coal but provided the biomass is derived from a source that is constantly replenished by regrowth. Then the new biomass that grows will absorb carbon dioxide from the atmosphere equivalent to that released during combustion.
Coal-fired power plants will burn biomass in exactly the same way as they burn coal. Depending on the amounts involved, the plant may require modification. Small quantities of biomass, up to perhaps 7%, can be simply mixed with the coal that is used to fire the plant without any adaptation being necessary. Pulverized coal and pulverized biomass enter the combustion chamber together and there is little change to operating conditions.
For the proportion of biomass to be increased beyond this, dedicated bio- mass burners must be used so that the conditions within the furnace can be more carefully controlled. With this technique up to 20% biomass can be introduced into a modern supercritical PC plant without adversely affecting its operation. This is also an advantageous means of using biomass because the efficiency of a modern coal-fired power plant will be much higher than of a dedicated biomass plant.
It is also possible to gasify biomass, generating a low-calorific-value fuel that can also be burned in a furnace. Introducing this gas rather than solid bio- mass into a coal-fired plant may offer some operational advantages. However, it does require a gasifier, making it more expensive and technically more complex than simple biomass cofiring.
Coal Gasification
The most straightforward approach to eliminating the carbon emissions result- ing from coal combustion in a power station is to capture the carbon dioxide produced in a chemical absorption plant. This is similar in concept to an FGD scrubber for removing sulfur dioxide. Carbon dioxide capture is carried out today in a number of industries using a reagent called monoethylamine (MEA) and the process has even been applied on a small scale at a number of power plants to provide pure carbon dioxide for the foods industry. Ammonia and other amine reagents can also be used to capture carbon dioxide and this is a fertile area for research with a wide range of alternative absorbents and techniques being studied. These include cryogenic capture, solid rather than liquid absorbents, and membrane systems.
The key to any reagent for carbon dioxide capture is that the chemical reaction must be easily reversible so that the carbon dioxide can be released again and the reagent regenerated. Ease of capture depends on the concentration of carbon dioxide, which in the flue gases of a typical PC power plant will be around 14% by volume. At this concentration some form of chemical absorption process is necessary to achieve the required level of capture efficiency. This makes the regeneration more energy intensive than would be the case with a physical absorbent. Amines appear to be the best reagents available today but others may become available in the future.
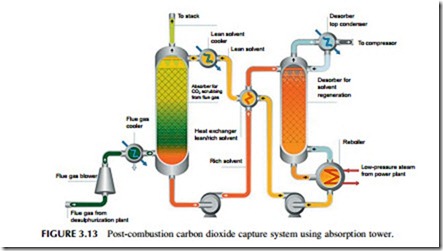
In a typical post-combustion capture plant the flue gases from the boiler are passed up a tall tower into which the reagent is sprayed so that gases and the liquid absorbent mix thoroughly (Figure 3.13). Depending on the column size, around 90% of the carbon dioxide within the flue gases can be removed in this way. The MEA reagent that has been sprayed into the path of the flue gases is collected at the bottom of the tower and then pumped to a second unit that heats it, releasing the carbon dioxide and returning pure MEA to enter the scrubbing tower once again. The regeneration process is extremely energy intensive, taking around 23% of the power output of the power station. A further 8% of the
output is then needed to compress the carbon dioxide so that it can be trans- ported for sequestration, and the MEA absorption plant uses a further 5% of the plant output as auxiliary power. This reduces the overall efficiency of power generation significantly. For example, an ultra-supercritical PC power plant that might achieve an efficiency of 45% would have this cut to around 35% with post-combustion capture. This has a large effect on the cost of electricity from such a plant.
Aside from its relative simplicity, one of the attractions of post-combustion carbon capture is that it can be applied retrospectively to existing power stations. This is unlikely to be cost effective for a large part of the old fleet of sub- critical coal-fired power plants operating today, but it could be economical to retrofit newer and more efficient supercritical plants.
Oxy-fuel Combustion
When coal is burned in air, the carbon dioxide resulting from the combustion reaction is mixed with a large quantity of nitrogen along with a number of other constituents of air, pollutant gases, and particles generated during combustion. The carbon dioxide accounts for only around 14% of the mixture and removing this low concentration is relatively difficult. If, on the other hand, coal is burned in pure oxygen, then the flue gases resulting will be almost pure carbon dioxide. This will be mixed with the little nitrogen left over after oxygen purification, some water vapor, and the various pollutants generated. Isolating carbon dioxide from this mixture is much simpler than in the case of air combustion. This is the concept behind oxy-fuel combustion.
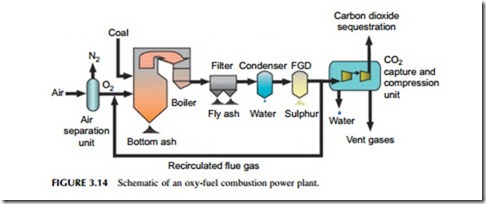
Oxy-fuel combustion essentially swaps a carbon dioxide separation plant for an oxygen separation plant so the efficiency of this plant now becomes key to
the overall efficiency (Figure 3.14). Oxygen separation plants are widely used industrially but it is an energy-intensive process. Reducing the energy cost is likely to be important if the technology is to become successful.
Oxy-fuel combustion is not simply a matter of replacing the air with oxygen for combustion in a coal-fired plant. Other changes must be made too. When coal burns in oxygen the flame temperature can reach 2500 oC, far higher than current materials can stand. To overcome this, some of the carbon dioxide–rich flue gas from the exhaust of the boiler must be fed back and mixed with the oxygen, diluting it and reducing the flame temperature in the process.
Energy conversion efficiency for an oxy-fuel combustion PC plant appears to be slightly lower than for a plant with post-combustion capture; estimated efficiencies are in the range 29–32%, but since the technique has not yet been tested at a commercial scale, this remains speculative. However, like post- combustion capture, it is feasible to retrofit oxy-fuel combustion to an existing coal-fired power plant. Again this is only likely to be effective with a high- efficiency supercritical plant, but it does offer an interesting alternative for future carbon capture.
Biomass Cofiring
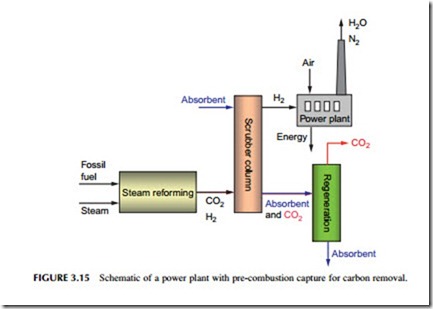
As noted before, coal gasification is another well-established industrial technique, used both as a source of domestic gas in the past and in the chemical industry to convert coal into a gas called synthesis gas or syngas that can be used as a precursor for a variety of chemical processes (Figure 3.15).
Coal gasification is carried out in a reactor called a gasifier where the fuel is burned under carefully controlled conditions with a strictly limited amount of either air or oxygen, together with steam. Oxygen gasifiers require a dedicated oxygen plant. The partial combustion of the coal, according to the reaction shown earlier, results in a gas that is primarily a mixture of hydrogen and carbon monoxide together with various combustion impurities such as hydrogen sulfide. A residual char is left.
Syngas still contains carbon in the form of carbon monoxide, so to create a carbon-free gas this must be passed through a second reactor where it is mixed with further steam and passed over a catalyst that converts the carbon monoxide into carbon dioxide and further hydrogen:
This process is called the water-shift reaction.
This mixture of carbon dioxide and hydrogen must now be cleaned and then the carbon dioxide separated. The latter is in much higher concentration than in the flue gases of a coal-fired power plant, accounting for around 50% of the mixture, and it can be separated using a physical absorbent rather than one that reacts chemically with the gas. The physical absorbent binds the carbon dioxide more loosely than a chemical absorbent such as MEA would, so releasing the carbon dioxide and regenerating the solvent is much less expensive from an energetic point of view. Reagents based on glycol and refrigerated methanol are used industrially to separate hydrogen from carbon dioxide, and these could be applied in a power plants based on gasification too.
To produce electricity from coal in this way, the hydrogen is burned in a gas turbine–based combined cycle power plant that is closely integrated with the gasification process to achieve the highest efficiency possible. This configuration is a variation of the IGCC power plant already described. As with the other approaches to carbon dioxide capture and removal, this leads to a significant fall in efficiency, and an IGCC plant with carbon capture appears likely to have an
energy conversion efficiency of around 32%, broadly similar to the other technologies discussed earlier.
An alternative to an IGCC plant is to use the hydrogen produced by the gasification process as fuel for a fuel cell. These devices, which are potentially capable of high efficiency when operating with pure hydrogen, will be discussed in a later chapter.
Related posts:
Incoming search terms:
- Coal-Fired Power Plant manufactures
- Coal Gasifier Power Plant manufactures
- Biomass Fired Power plant waste to energy equipment Gasifier manufactures
- Liquid carbon dioxide (LCO2) plant manufactures
- power plant carbon dioxide removal
- power generation plant that removes CO2
- environmental protection coal gasifier manufactures
- Durable oxygen Coal gasifier for gas generation plant manufactures
- Coal Gasifier-Coal Gasifier Plant-Coal Gasifier Power Plant manufactures
- 400kw biomass gasifier generator power plant manufactures
- coal gasifier manufactures
- CO2 concentration in lue gas from power plant
- Carbon dioxide removal and purification manufactures
- Carbon capture manufactures
- biomass gasification plant manufactures
- 500KW-1MW-5MW Steam Turbine Generator Biomass Gasification Power manufactures
- Wind Power Plant manufactures