FUEL CELLS
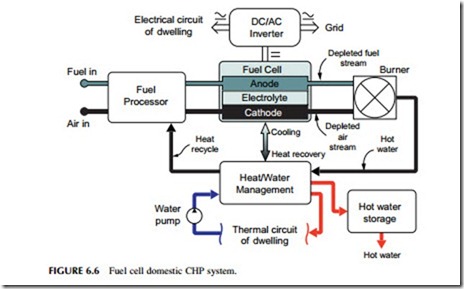
Fuel cells are electrochemical devices similar to batteries that convert the chemical energy in a fuel directly into electricity (Figure 6.6). Since fuel cells are not limited by the Carnot cycle efficiency, they are potentially more efficient than heat engines. However, very high efficiencies are difficult to realize in practice. Proton exchange membrane (PEM) fuel cells, being developed extensively for automotive applications, can achieve between 25% and 35% efficiency when operating on natural gas, although in principle these cells can run much more efficiently if they are supplied with hydrogen fuel. PEM cells operate at a relatively low temperature. High-temperature fuel cells, such as molten carbonate
and solid oxide fuel cells, can achieve over 40% efficiency and potentially up to 50% or higher. They are also capable of supplying high-grade heat. However, the most popular fuel cell for CHP applications is the phosphoric acid fuel cell (PAFC). Fuel cells are particularly good at load following as efficiency varies little with output.
The PAFC was one of the first fuel cells to be developed commercially. It operates at a moderate temperature of 150–200 oC, allowing it to produce low-grade heat for hot water and space heating. In common with most fuel cells, the PAFC requires hydrogen and oxygen to be supplied to its electrodes. Oxy- gen is provided from air, but in most applications hydrogen must be generated from natural gas in a process known as reforming. This is relatively energy intensive and reduces overall efficiency. Even so, the units can generate elec- tricity at an efficiency around 42%.
PAFC fuel cells are packaged so they can be installed rapidly and easily, requiring only a gas supply and connections for electricity and hot water output. Typical electrical generating capacities are 100–400 kW. In CHP applications they can achieve up to 87% efficiency. Units are virtually noiseless and emissions are generally negligible making them easy to site. The range of electrical outputs available from single units makes them most suited to small commercial and institutional applications, such as schools, hospitals, and offices.
PEM fuel cells operate at a much lower temperature than PAFC fuel cells, usually around 80 oC. This means they can provide some hot water, and small units are being built aimed at the residential market. Electrical efficiency for such systems is around 30% and CHP efficiency 80%. Unit sizes can range from
3 kW to 250 kW. As with PAFC fuel cells, these cells require hydrogen to be supplied to their electrodes. Again this is normally produced by reforming natural gas.
Two main types of high-temperature fuel cells are under development: the molten carbonate fuel cell (MCFC) and the solid oxide fuel cell (SOFC). MCFC cells operate at around 650 oC and can achieve an electrical conversion efficiency of 47%. SOFC cells generally operate at a higher temperature, 750– 1000 oC, and have shown conversion efficiencies up to 43%. While both can be exploited for CHP applications, the MCFC is a relatively complex cell and has been developed for electricity production alone. The SOFC is simpler and some small SOFC units are being developed for domestic CHP applications. Capacities can range from as low as a few tens of watts to several megawatts.
Costs for all types of fuel cells remain high compared to other sources of electricity generation, but potential for cost reduction exists, particularly for PEM cells, which are being actively developed as power units for electric vehicles.