Physics of Carburetion
All automobile engines require a fuel charge in the engine cylinders. This fuel charge is a mixture of air and a vapor obtained from gasoline. The gasoline is atomized (broken up) , partially vapor ized and mixed with the correet proportion of air in the carburetor. To understand the mechanics of a carburetor it is first necessary to understand the principles of carburetion.
WEIGHT OF AIR
It is not generally appreciated that air has weight and that this weight decreases as the height above sea level increases. The weight of air (atmospheric pressure) has a definite bearing on carburetor design, construction and adjustment. Mechanics are most interested in adjustmen t, since it varies with each grade of gasoline, with various load conditions and with different alti tudes.
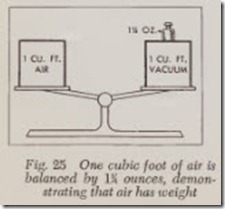
To show that air has weight, two identical air tight vessels, each containing 1 cubic foot of air are placed on a balance as shown in Fig. 25. If all the air is extracted from one vessel with a vacuum pump, it will weigh B ounces less than the other.
A cubic foot of air weighs B4 ounces, but a cubic foot of gasoline weighs about 775 ounces ( 483 pounds). This means that a cubic foot of air will have to be multiplied by 620 to equal the weight of a cubic foot of gasoline.
The ideal air-fuel ratio for an automobile engine is 15 parts of air to 1part of gasoline. Accordingly, to burn 1 cubic foot of gasoline, 15 times 620 or 9,300 cubic feet of air is needed. Converting this ratio to gallons: 9,000 gallons of air is needed to burn 1 gallon of gasoline the air-fuel ratio is 9000: 1 by volume. To put it another way, Y:z teaspoonful of gasoline has to be mixed with 1 cubic foot of air to obtain the ideal15 to 1ratio.
VAPOR IZATION
Jn order to secure a good air-fuel mixture, gasoline must first be atomized (broken up) and vaporized. If 3 teaspoonful of gasoline is poured into a cubic foot of air the gasoline simply drops to the bottom of the vess el and remains there without mixing with the air except by the slow process of evaporation.
Vapor is a gasified liquid. When a liquid vapor izes it occupies more space, and it will also :Boat in air. Evapor ation occurs at all temperatures, but it is more rapid in hot weather. All volatile liquids placed in contact with the air will start to evapo rate and in this vapor form may present extremely dangerous fire and explosion hazards. The loss from evaporation is consid erable, especially in extremely warm weather, and must be considered when handling fuels in bulk. As a rule, the evapo rated p articles are not visible, but in most cases the vapors from an evaporated gasoline give off an odor which may aid in detecting abnormal evapo rating conditions .
It is poss ible to accelerate vaporization of a fuel by a number of methods. The vaporization of a gasoline in an automobile engine is carried out progressively The breaking up of a fuel is started by the action of the needle valve and the venturi tubes . It is partia lly vaporized and atomized by the su ction pr oduced in the top of the venturi tube. It is further vaporized as it passes through the intake manifold, by the heat transferred to the manif old from the engine, and it is almost com pletely vaporized durin g the compression stroke of the piston by the heat of compression and the heat left by the previously burn ed fuel. In carbu retor design, the practice is to start vaporization as soon as possible.
Vaporization by Spraying
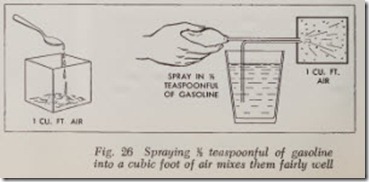
If an atomizer is used for vaporizing a fuel, as shown in Fig. 26, it is possible to spray tea spoonful of gasoline into a cubic foot of air very quickly and have it mix fairly well, since it enters the air in the form of a mist. The use of an atomizer represents one of the most important principles of carburetion. When the bulb is pressed, the suction created at the nozzle by the expelled air draws portions of the gasoline up through the tube. As it draws the gasoline out, the air from the bulb strikes it and breaks it up into small particles, or in other words, atomizes it. “Atom” is a term applied to very small parts of any element and atomization means breaking into small particles.
Vaporization by Heating
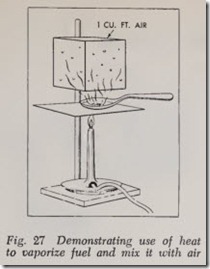
A liquid can be vaporized by applying heat to it. The greater the heat, the more rapid the vapor ization. If heat is applied to teaspoonful of gasoline, as shown in Fig. 27, the gasoline will be driven off very quickly in the form of gasoline vapors. This vapor rises and if the vessel contain ing the cubic foot of air is immediately above it the air and vapor mix fairly well.
This heating principle is made use of in carbu retors by several methods. The older method was to make use of a “stove” built around the exhaust manifold at a point close to the carburetor so that the air being drawn into the carburetor was first passed around the manifold and heated. While stoves are still used to a limited extent, the mod ern practice is to use the “hot spots” around the manifold passages (see Fig. 18). The atomized gasoline, as it passes from the carbur etor into the manifold, comes in direct contact with a heated surface and vaporizes rapidly.
Vaporization by Vacuum
The earth is surrounded by an ocean of air, many miles in height, that presses upon the earth with a pressure of 14.7 pounds per square inch at sea level. Atmospheric pressure is exerted in all directions. Air is continually forcing its way into any place that contains no air; that is, air will always try to fill a vacuum. Of course, the term “vacuum” means a lower air pressure than atmos pheric pressure.
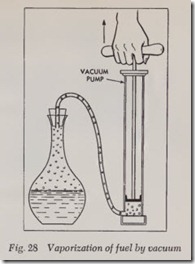
Air can be forced from a bottle half filled with water by connecting it to a vacuum pump, as shown in Fig. 28. As pressure is lifted from the water, it begins to vaporize and it will then boil at 80 to 90 degrees instead of the normal 212 degrees F. If half the air is removed from a sealed container, the air left will expand as the pressure is removed, leaving th e air pressure within the con tainer only about half that of the atmospheric pressure on the outside of the container. This unequal pressure speeds up the vaporization action.
Vaporization by vacuum has a very important place in carburetor operation. A vacuum is the principal means of drawing fuel into the mixing chamber of a carburetor. A venturi tube , which is located just ahead of the mixing ch amber, is used in combination with the suction of the engine to increase the vacuum. The lowered pressures due to the vacuum in the venturi allow the atomized fuel to expand and vaporize more quickly.