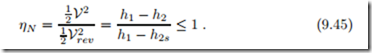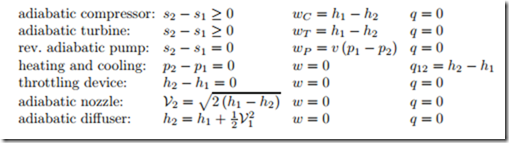Isentropic Efficiencies
Many thermodynamic processes are adiabatic, since they happen so fast that no heat can be exchanged during the process. When the process is adiabatic and reversible, the second law tells us that the process is isentropic as well, since Q˙ = 0 and S˙gen = 0. Real processes, however, will always be irreversible, so that real adiabatic processes are accompanied by entropy generation. A useful measure for the quality of the performance of irreversible devices is given by efficiency measures that compare adiabatic irreversible devices to their isentropic counterparts. In the following we discuss isentropic efficiencies for compressors, pumps, turbines, nozzles and diffusers.
Compressor: The left T-s-diagram in Fig. 9.6 shows, for an ideal gas, two isobaric lines. An adiabatic and reversible compression of the gas between pressures p1 and p2 follows the isentropic path 1-2s; the required compressor work per unit mass is wC,rev = h1 − h2s. A real compressor is irreversible due to internal friction and internal heat transfer, and the compressed state will have a larger entropy. The endpoint of the compression is point 2 with s2 > s2s = s1 and the required compressor work is wC = h1 −h2. Note that in the figure the line connecting points 1 and 2 is dotted to indicate that during compression the gas is in non-equilibrium states that cannot be marked as a path in the T-s-diagram, only the initial and endpoints are known.
The performance of an adiabatic compressor can be measured by comparing its actual work requirement with the best case by means of the isentropic compressor efficiency
The real compressor requires more work input, since some work is needed to overcome friction and other irreversibilities. The isentropic efficiency is defined such that it has values between 0 and 1; realistic compressors have efficiencies ηC = 0.8 − 0.9. This definition can be extended to pumps, which do not compress a gas, but increase the pressure in a liquid.
Turbine: The expansion in an adiabatic and reversible turbine follows the isentropic path 1-2s in the right T-s-diagram of Fig. 9.6; the work delivered per unit mass is wT,rev = h1 − h2s. A real turbine is irreversible, and will expand to state 2 producing the work wT = h1 − h2.
The performance of an adiabatic turbine can be measured by comparing its actual work requirement with the best case by means of the isentropic turbine efficiency
The real turbine delivers less work, since some work is consumed to overcome friction and other irreversibilities. The isentropic efficiency is defined such that it has values between 0 and 1; realistic turbines have efficiencies ηT = 0.85 − 0.95.
Nozzle: An adiabatic nozzle accelerates the flow, while no work is exchanged. For a nozzle expanding from p1 to p2, the outflow velocity is V = …2 (h1 − h2)
(inflow velocity ignored). The nozzle efficiency is usually defined not by the velocity, but the specific kinetic energy of the outflow, as
Typical nozzle efficiencies are above 95%.
Diffuser: Isentropic diffuser efficiency cannot be defined through enthalpies that easily. The first law for the diffuser states h2 = h1 + 1 V2, which implies that for an ideal gas, where h2 = h (T2), the exit temperature T2 is the same for reversible and irreversible diffusers. However, irreversibilities lead to lower exit pressure p2 as compared to the reversible exit pressure p2s, see the T-s-diagram in Fig. 9.9. Not all of the available kinetic energy 1 V2 is used for compression, i.e., pressure increase, but some is lost to irreversibility. A reversible diffuser that gives the end pressure p2 of the irreversible diffuser would convert the kinetic energy 1 V2 = hx − h1, where hx = h (Tx), and Tx is the temperature at the end of an isentropic compression between the inlet state {p1, T1} and {p2, Tx}. With the help of this artificial state, isentropic diffuser efficiency can be defined as
Summary: Open System Devices
For later reference, we list work, heat and operating conditions for the most common one-inlet-one-exit devices in a table: