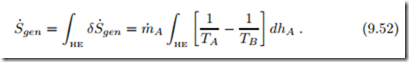Closed Heat Exchangers
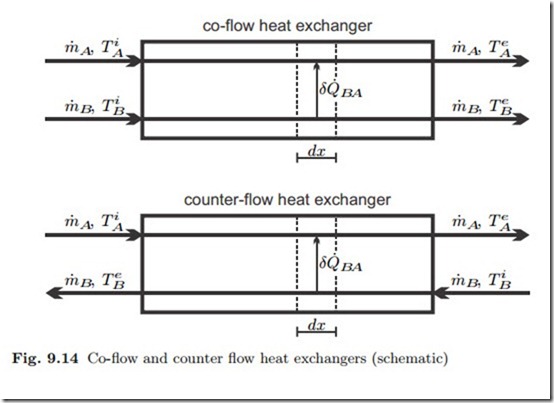
Closed heat exchangers serve to transfer heat between different fluids, for instance from the hot combustion gas to evaporating water in steam power plants, or from the condenser in the power plant to the cooling water. We ignore friction losses in the flow, so that the flows are isobaric. Figure 9.14 shows the exchange of heat between two fluids A and B in co- and counter- flow heat exchangers, where superscripts i and e denote incoming and exiting flows, respectively. Since the fluids flow through different piping systems, they might have different pressures pA, pB .
The conservation laws for mass and energy give for both set-ups (adiabatic to the outside, steady state)
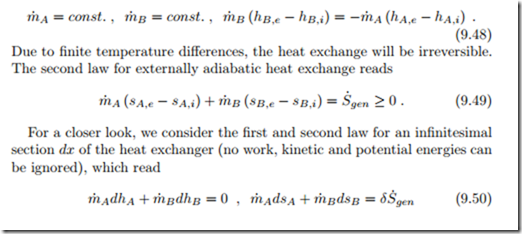
For a closer look, we consider the first and second law for an infinitesimal section dx of the heat exchanger (no work, kinetic and potential energies can be ignored), which read
For isobaric processes the Gibbs equation gives dh = T ds, which allows to combine the above to
Accordingly, entropy generation and lost work depend on the local temper- ature difference between the streams. The total entropy generation is given by the integral over the length of the heat exchanger (HE),
To see that the entropy generation is positive, we note that, under the given simplifications, the first law for stream A reads m˙ AdhA = δQ˙ BA where δQ˙ BA is the heat going from B to A (heat into the system is positive). Since heat goes from warm to cold, we have δQ˙ BA > 0 if TB > TA .
A detailed discussion of counter-flow and co-flow heat exchangers shows that the local temperature difference between the two flows is smaller for the counter-flow design, which therefore is thermodynamically preferable (see Sec. 15.2).
Open Heat Exchangers: Adiabatic Mixing
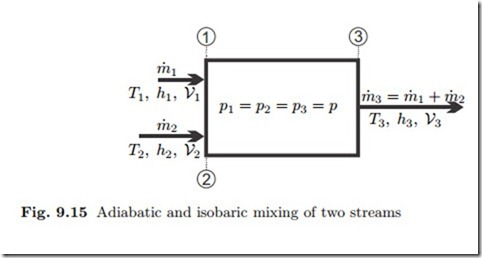
Another interesting process that occurs quite often is heat exchange be- tween two flows by adiabatic mixing of two (or more) streams, as depicted in Fig. 9.15. To prevent backflow, the mixed streams should have the same pressure, but their temperatures and/or phase states are different. For the computation, pressure losses through the mixing chamber are normally ignored, so that the outflow pressure equals the inflow pressure. Moreover, kinetic and potential energies can be ignored since their changes are much smaller than changes in enthalpy.
That is, the mass flow and the total enthalpy flow are conserved in mixing. Since mixing occurs uncontrolled, the process is irreversible, and the entropy will increase; from (9.13) we find
There is a corresponding work loss, since the temperature difference between the flows could be used to drive heat engines, but is not.
Related posts:
Incoming search terms:
- open and closed type heat exchanger
- closed heat exchanger
- what is open flow heat exchanger
- Propylene Glycol – Closed Loop HVAC mail
- process to open heat exchanger
- open type heat exchanger
- open loop vs closed loop heat exchanger
- open flow heat exchanger
- is continues heat exchanger open or close system
- heat exchangers for open to closed systems
- heat exchanger is open or closed system
- heat exchanger is open or close system
- What Is Open Type Exchanger