Although it is a commonly held belief, it is incorrect to regard air- conditioning as simply the cooling of air. Conditioning the air of a space means to change it in whatever way necessary to improve the comfort of those living or working there. This may mean warming air to a livable temperature and holding it there; cooling the air; adding or subtracting moisture; filtering out contaminants such as dust, bacteria, and toxic gases; and maintaining a proper distribution and movement of the air. In general, air-conditioning includes the following processes:
1. Heating
2. Cooling
3. Humidification
4. Dehumidification
5. Cleaning and filtering
6. Air movement (circulation)
Each of these processes contributes in some way to the improvement of those conditions that affect the comfort and health of an individual. For example, air nearly always contains certain impurities, such as ammonia, sulphurous acid, and carbon dioxide. The last named is a product of exhalation from the lungs and the combustion process in internal combustion engines. It is so universally present (about in the same proportions everywhere, except where concentrated by some local conditions) that it may be regarded as a normal part the air. Air-conditioning is an efficient means of eliminating carbon dioxide from the air. The same is true of other impurities. Some dry strainer filters used in air-conditioning systems are capable of removing 99.98 percent of radioactive dust from the air.
To understand how an air-conditioning system works and how to calculate cooling loads, you should have a basic understanding of the physical properties of air and how its moisture content, temperature, and pressure will influence your calculations. These topics and the associated terminology are described in the following sections.
Properties of Air
Air is composed of water vapor and dry air. These two components are combined in such a way that neither loses its distinct characteristics. A number of different terms are used to describe the qualities or properties of air, but the two terms essential in heating and cooling calculations are humidity and temperature.
Humidity
Humidity is a general term used to refer to the water vapor (moisture) content of air. When this term is used, it is usually in reference to the sensation (or lack of sensation) of moisture in the air. For purposes of heating and cooling calculations, the more narrowly defined terms of absolute humidity, relative humidity, and specific humidity are used.
Water vapor is actually steam at low temperatures and, consequently, low pressures; hence its properties are those of steam. According to Dalton’s law, in any mechanical mixture of gases, each gas has a partial pressure of its own, which is entirely independent of the partial pressures of the other gases of the mixture.
Note
In all air-conditioning calculations it should be understood that the dry air and water vapor composing the atmosphere are separate entities, each with its own characteristics. Water vapor is not dissolved in the air in the sense that it loses its own individuality and merely serves to moisten the air.
Air is capable of holding, as a mechanical mixture within itself, varying quantities of water vapor, depending on its temperature. When air absorbs moisture; that is, when it is humidified, the latent heat of evaporation must be supplied either from the air or from another source. And conversely, when the moisture from the air is condensed, the latent heat of condensation (equivalent to the latent heat of evaporation) is recovered.
Air is said to be saturated when it contains all the water vapor it can hold. If partly saturated air is reduced in temperature until the amount of moisture present corresponds to the amount that the air is capable of holding at the given temperature, it will become saturated air.
If the temperature of the air is still further reduced, its ability to hold moisture will be reduced accordingly. As a result, the excess moisture will be condensed, which means that it will be converted from a vapor to a liquid. This is the reverse of the process that occurred as the air absorbed the moisture.
Converting liquid water into water vapor requires a great quantity of heat. The heat necessary for this process is used only in per- forming the conversion, the temperature of the liquid and the vapor being the same at the end of the process. If the conversion is from liquid to vapor, this involves the latent heat of evaporation. The latent heat of condensation is involved if the conversion is from vapor to liquid.
Cold air is saturated when it contains very small quantities of water vapor, whereas warm air is not saturated until it contains larger quantities of vapor. For example, air at zero degrees Fahrenheit is saturated when it contains but one-half of one grain (1/7000 lb) of water vapor per cubic foot. Air at 70°F is saturated when it contains 8 grains of vapor per cubic foot, while at 83°F, 12 grains per cubic foot are required to saturate.
Absolute Humidity
Absolute humidity is the actual mass of water vapor in one cubic foot of air (that is, the weight of water vapor per unit volume of air) and is expressed in grains or pounds per cubic foot (1 lb = 7000 grains), or grams per cubic centimeter. Absolute humidity is equivalent to the density of the air.
Specific Humidity
Specific humidity is the weight of water vapor per pound of dry air.
Do not confuse specific humidity with relative humidity. The latter term indicates the percentage of water vapor, the former the weight.
Relative Humidity
Relative humidity is the ratio of absolute humidity to the maximum possible density of water vapor in the air at the same temperature. In other words, it is a percentage or ratio of water vapor in the mix- ture of dry air and water vapor at a certain temperature relative to the maximum quantity that the volume of air could possibly carry at that temperature. The relative humidity at any given temperature can be obtained by first using a sling psychrometer to determine the amount of moisture (that is, water vapor) actually present in the air and then dividing this figure by the amount of moisture that the air can hold at that temperature, and multiplying the result by 100 in order to obtain the percentage factor.
Drying Effect of Air
The drying effect of air varies approximately inversely with its relative humidity. In other words, the drying effect decreases as the relative humidity increases. It should be noted that it is relative humidity that determines the drying effect of air, and this effect depends on both the temperature and the water content of the air since relative humidity depends on both these factors.
The quantity of heat that dry air contains is very small because its specific heat is low (0.2415 for ordinary purposes), which means that 1 lb of air falling 1°F will yield only 0.2415 of the heat that would be available from 1 lb of water reduced one degree in temperature. The presence of water vapor in the air materially increases the total heating capacity of the air because of the latent heat of the vapor itself.
Most hygroscopic materials in the presence of dry air, even at high, dry-bulb temperatures, may actually be cooled rather than heated. This occurs because the dry air immediately begins to evaporate moisture form the material.
The Dew Point
The dew point is the temperature of saturation for a given atmospheric pressure. In other words, for a given atmospheric pressure (barometer), it is the temperature at which moisture begins to con- dense in the form of tiny droplets, or dew.
The saturation temperature for any given quantity of water vapor in the atmosphere is known as the dew point. Any reduction in temperature below the dew point will result in condensation of some of the water vapor present, with a consequent liberation of the latent heat of the vapor, which must be absorbed before any fur- ther condensation can take place.
If the vapor pressure of the water vapor in a given space is the same as the vapor pressure of saturated steam at the prevailing dry- bulb temperature, the space contains all the water vapor it can hold at that temperature. The term saturated water vapor is applied to water in this state.
Humidification
Humidification may be defined as the addition of moisture to the air. The conditioning machine that functions to add moisture to the air is called a humidifier. A humidifier is commonly a low- pressure, low-temperature boiler in which the water is evaporated and the vapor (low-pressure steam) thus formed is caused to mix with air.
In a sense, water functions as a natural humidifier by acting as the medium that conveys heat to the air and as the source of the water vapor required to saturate the heated air. Contrast this with what takes place in a humidifier unit. A machine functions as a humidifier when the temperature of the spray water is above that at which the moisture in the air will condense.
Dehumidification
Dehumidification may be defined as the removal of moisture from
the air. A machine that functions to remove moisture from the air is called a dehumidifier.
The removal of moisture from the air is accomplished by condensation, which takes place when the temperature of the air is lowered below its dew point. The condensation thus formed falls into the tank of the conditioning machine. In this case the water acts solely as a conveyor of heat from the air (in addition to its cleansing action) and, as such, the finely divided mist is extraordinarily effective (practically 100 percent).
Some conditioning machines can function both as humidifiers or dehumidifiers. This can often be done without alteration to the unit except that the valves in the control line from the dew point ther- mostat on some designs are adjusted to connect the steam control of the water heater for winter operation, and to connect the three- way mixing valve to the water supply line for summer operation.
Whether the requirement is humidification or dehumidification, the unit always operates under accurate automatic control, main- taining the required indoor conditions winter and summer, regardless of the outdoor weather.
Temperature
Temperature is a general term used to describe the sensation (or lack of sensation) of heat in the air. Among the more specific terms used in the heating and cooling calculations to describe the air temperature are dry-bulb temperature and wet-bulb temperature.
Note
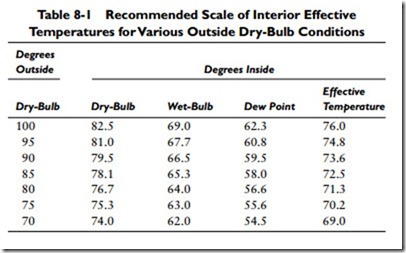
Sometimes both temperature and humidity are used in conjunction with one another as a calculation factor, and the tempera-turehumidity index is an example of this. By definition, the temperature-humidity index (formerly called the discomfort index) is a numerical indicator of human discomfort resulting from temperature and moisture. It is calculated by adding the indoor dry-bulb and the indoor wet-bulb thermometer readings, multi- plying the sum by 0.4 and adding 15. The results you obtain are the same as those used for the effective temperature index (see Standards of Comfort). This can be worked out from the data provided in Table 8-1.
Dry-Bulb and Wet-Bulb Temperatures
Dry-bulb temperature is the actual temperature of air as measured by an ordinary thermometer. Wet-bulb temperature is the temperature at Table 8-1 Recommended Scale of Interior Effective Temperatures for Various Outside Dry-Bulb Conditions
which the air would become saturated if moisture were added to it without the addition or subtraction of heat. It is the temperature of evaporation. In actual practice, the wet-bulb temperature reflects humidity conditions in the area. A high wet-bulb reading, for example, means that the humidity is also high.
The wet-bulb temperature in conjunction with the dry-bulb temperature is an exact measure of both the humidity of the air and its heat content. In air-conditioning the dry-bulb temperature and the wet-bulb temperature must both be controlled if the effects of air are to be regulated.
If the bulb of an ordinary thermometer is surrounded with a moistened wick and placed in a current of air and superheated water vapor, it will be found that a reading at some point below the dry-bulb temperature is obtained. The minimum reading thus obtained is the wet- bulb temperature of the air. The reduction in temperature is caused by the sensible heat being withdrawn from the air to vaporize the water surrounding the wet bulb, thus raising the dew point of the air.
Note
The point of equilibrium at which the withdrawn sensible heat balances with the heat of vaporization necessary to bring the dew point up to the same point is the wet-bulb temperature.
In this transformation of energy from sensible heat of vaporization, there is no change in the total amount of energy in the mixture. For this reason, the wet-bulb temperature, once fixed, is an indication of the total heat in any mixture of air and water vapor.
The daily temperature range is the difference between the maxi- mum and minimum dry-bulb temperatures during a 24-hour period on a typical day for a heating or cooling system. It is used in deter- mining the factors used in making Btu tabulations. The Btu tabulation cooling form illustrated in Figure 8-9 shows their use. In Figure 8-9 you will note that the tables labeled “wall factors” and “ceiling factors” each have a column reserved for four different degrees of daily temperature range (that is, 15°F, 20°F, 25°F, and 30°F). Reading across from left to right, the different daily temperature ranges intersect with a number of other columns representing differences in the dry-bulb temperatures. The selection used will depend on the type (or absence) of insulation.
Wet-Bulb Depression
Because outdoor summer air is rarely fully saturated, there is usually a considerable difference between its dry-bulb and its wet-bulb temperatures. This difference is referred to as wet bulb depression and is greatest during the summer.
As previously mentioned, the wet-bulb temperature is that temperature to which air would be cooled by evaporation if the air was brought into contact with water and allowed to absorb sufficient water vapor to become saturated. For example, if the outdoor summer air is drawn through a humidifier and completely saturated, its dry-bulb temperature will be reduced to its wet-bulb temperature, and the air will leave the humidifier at the outdoor wet-bulb temperature. This cooling is accomplished entirely by evaporation and is due to the latent heat required to convert the liquid water vapor. This conversion occurs the instant the air is brought into contact with the mist in the spray chamber of the humidifier, the heat being taken from the air.
The spray water in a humidifier is used over and over again, only that quantity being added which is actually absorbed by the air. Thus, without any additional expense, a humidifier will perform the function of cooling the air through the wet-bulb depression in the summer.
The extent of the wet-bulb depression in some localities is as much as 25° or 30°. Even in localities adjacent to large bodies of water where the humidity is high and the wet-bulb depression correspondingly low, the latter will quite commonly range from 10° to 15°.
In the vicinity of New York, for instance, the maximum outdoor wet-bulb temperature is about 78°. On such a day the dry-bulb temperature would probably be about 90°, making the wet-bulb depression 12° (90° 78° = 12°).
In Denver, on the other hand, the maximum outdoor wet-bulb temperature is usually less than 78°. Because the coincident dry- bulb temperature is usually much higher then 90°, it results in a
greater wet-bulb depression, which means that more cooling can be accomplished by evaporation.
Sensible and Latent Heat
The distinction between dry air and the moisture content of air and between dry-bulb and wet-bulb temperatures is extended to the two types of heat carried by the entering air and the air already contained in the space: sensible and latent heat.
Sensible Heat
Sensible heat is the amount of heat in air that can be measured by an ordinary thermometer (that is, a dry-bulb thermometer). The daily weather report gives us sensible heat temperatures, but it does not represent the total heat we experience. It constitutes a portion of the heat resulting from air infiltration and ventilation, and internal heat sources such as people, electric lights, and electric motors. Sensible heat also results from heat leakage (or heat loss in the case of heating calculations) and solar radiation.
Latent Heat
Latent heat is the amount of heat contained in the water vapor (moisture) of the air. It constitutes a portion of the heat resulting from infiltration and ventilation and any internal sources capable of adding water vapor to the air (for example, cooking vapors, steam, people). The amount of latent heat in the air can be deter- mined by using a psychrometric chart (see Appendix E, “Psychrometric Charts”). The amount of excess latent heat so determined will indicate the amount of moisture that must be removed from the air in order to obtain comfortable conditions.
Both sensible heat gain and latent heat gain are expressed in Btu. When the total of the two are added together, their sum represents the total heat gain in Btu that must be removed from the air each hour by the air conditioner.
Sensible heat gain (or load) is represented by a change in the dry- bulb temperature readings, whereas latent heat gain is represented by a change in the web-bulb temperature.
Pressure
Atmospheric air is air at the pressure of the standard atmosphere.
Standard atmosphere is considered to be air at a pressure of 29.921 inches of mercury, which is equal to 14.696 pounds per square inch (usually written 14.7 psi). In any air-conditioning system, atmospheric air is regarded as being air at the atmospheric pressure at the point of installation.
Standard atmospheric pressure at sea level is 29.921 inches of mercury. Since most pressure gauges indicate gauge pressure or pounds per square inch, a barometer reading can be converted into gauge pressure by multiplying inches of mercury by 0.49116 psi. Thus, a barometer reading of 29.921 inches is equivalent to a gauge pressure of 14.696 psi (29.921 X 0.49116 = 14.696, or 14.7 psi).
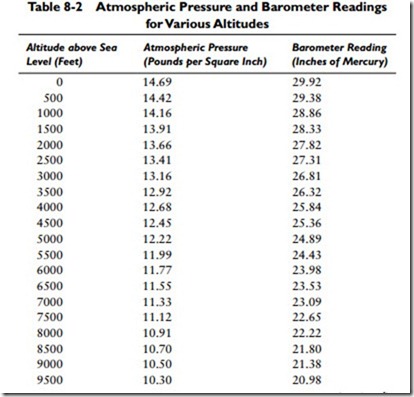
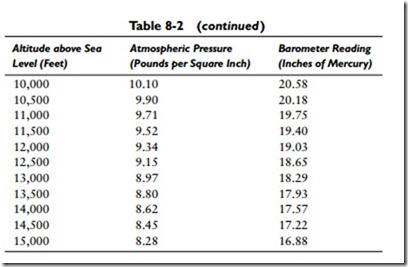
So 0.49116 psi is a constant value for 1 inch of mercury and is determined by dividing the pressure in pounds per square inch by the barometer readings in inches of mercury (that is, 14.696 –: 29.921 = 0.49116). Table 8-2 gives the atmospheric pressure for various readings of the barometer.
The pressure of the atmosphere does not remain constant in any one place. It continually varies depending on the conditions of the weather and the elevation. With respect to elevation, atmospheric pressure will decrease approximately 1⁄2 lb for each 1000 feet of ascent. Table 8-2 illustrates the effect of altitude and weather (the barometer reading) on atmospheric pressure.
Absolute pressure is pressure measured from true zero or the point of no pressure. It is important to distinguish absolute pressure from gauge pressure, whose scale starts at atmospheric pressure. For example, when the hand of a steam gauge is at zero, the absolute pressure existing in the boiler is approximately 14.7 psi. Thus, 5 lbs pressure measured by a steam gauge (that is, gauge pressure) is equal to 5 lbs plus 14.7 lbs, or 19.7 psi of absolute pressure.
Related posts:
Incoming search terms:
- absolute humidity in air conditioning
- what is absolute humidity with reference to air conditioning
- wet bulb temperature steam table calculation
- significance of dbt and wbt in air conditioning
- in winter air condioning WBT effect
- how to improve wet bulb temperature in air conditioner
- for comfort air conditioning what is dbt and wbt
- dry bulb temperature and wet bulb temperature of the air conditioned space
- Dry bulb temperature /air conditioning
- dry & wet bulb temp in air conditining
- diffeerent between absolute humidity and wet bulb temperature
- convert wet bulb to relative humidity
- air conditioner WBT
- Absolute humidity with reference to air conditioning
- व्हाट इस डिप्रेशन ऑफ़ वेट बल्ब