conduction in Pure Germanium and Silicon
The electrical activity in semiconductor material is highly dependent on temperature. At extremely low temperatures, valence electrons are held tightly to the parent atom through the covalent bond. Because these valence electrons cannot drift, the material cannot support current flow. Germanium and silicon crystals function as insulators at low temperatures.
As the temperature increases, the valence electrons become agitated. Some of the electrons break the covalent bonds and drift randomly from one atom to the next. These free electrons are able to carry a small amount of electrical current if an electrical voltage is applied. At room temperature, enough heat energy is available to produce a small number of free electrons and to support a small amount of current. As the temperature increases, the material begins to acquire the characteristics of a conductor. Only at extremely high temperatures does silicon conduct current as ordinary conductors do. Typically, such high temperatures are not encountered under normal usage.
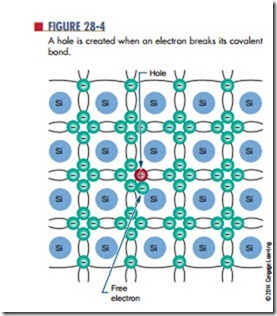
When an electron breaks away from its covalent bond, the space previously occupied by the electron is referred to as a hole (Figure 28-4). As described in Chapter 11, a hole simply represents the absence of an electron. Because an electron has a negative charge, its absence represents the loss of a negative charge. A hole thus has the characteristic of a positively charged particle. As an electron jumps from one valence shell to another valence shell with a hole, it leaves a hole behind it. If this action continues, the hole appears to move in the opposite direction to the electron.
Each corresponding electron and hole are referred to as an electron-hole pair. The number of electron hole pairs increases with an increase in temperature. At room temperature, a small number of electron-hole pairs exists.
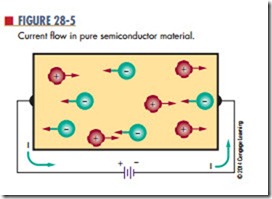
When pure semiconductor material is subjected to a voltage, the free electrons are attracted to the positive terminal of the voltage source (Figure 28-5). The holes created by movement of the free electrons drift toward the negative terminal. As the free electrons flow into the positive terminal, an equal number leave the negative terminal. As the holes and electrons recombine, both holes and free electrons cease to exist.
In review, holes constantly drift toward the negative terminal of the voltage source. Electrons always flow toward the positive terminal. Current flow in a semi- conductor consists of the movement of both electrons and holes. The amount of current flow is determined by the number of electron-hole pairs in the material.
Questions
1. How can pure germanium support a current flow?
2. Describe the process of electrons moving through semiconductor material.
3. When a potential is applied to pure germanium, in what direction do the electrons and holes move?
4. What happens when holes and electrons recombine?
5.What determines the amount of current flow in a pure semiconductor material?
Related posts:
Incoming search terms:
- Explain how pure germanium can be treated in such a way that conduction is predominantly due to holes
- conduction of pure silicon
- explain how pure germanium can be treated in such a way that conduction is predominantly
- how can you germanium support a current flow
- conduction in pure semiconductor
- pure germanium silicon are
- pure semiconductor produce electrical voltage at room temperature?
- when a potential is applied a germanium to pure germanium in what direction do to the electron and holes moves