Dehumidification
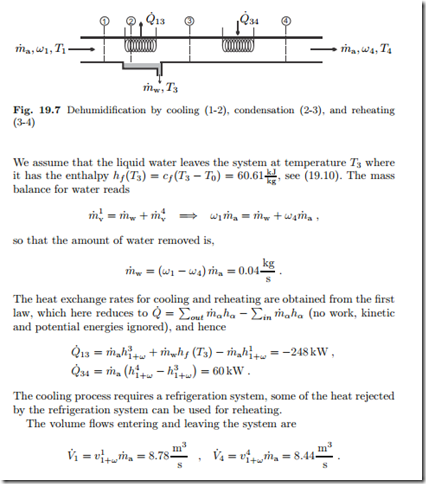
When moist air is cooled below its dewpoint, water condenses, and the humidity ratio ω drops. This process forms the basis for dehumidification systems, in which moist air is cooled below the dewpoint, some water condenses, and then the air is reheated, so that a desired final state is reached, Figure 19.7 shows a schematic for such a process.
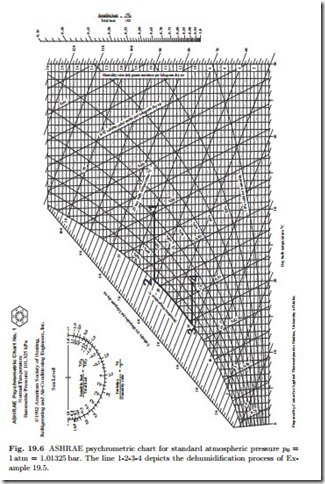
We study the process by means of an example, that also shows how to use the psychrometric chart. A mass flow m˙ a = 10 kg of outside air of state is to be dehumidified and cooled so that the final state 4 is T4 = 20 ◦C, φ4 = 0.7. To achieve this state, the flow is first cooled isobarically. As long as the temperature is above the dewpoint, the humidity ratio does not change. At state 2 (φ2 = 1), water starts to condense. The moist air is cooled further to state 3, while some water condenses. State 3 has the same humidity ratio as the desired final state 4, which is finally obtained by isobaric heating. The process curve is shown in the psychrometric chart, Fig. 19.6.
From the diagram, we read the following data for the process
Humidification with Steam
The humidity ratio of dry or moist air can be increased by adding water either as steam or as liquid at pressure p. Steam injection increases humidity ratio and temperature, as long as the steam temperature is above the air temperature. Injection of liquid water, e.g., by spraying of fine mist, leads to cooling of the air, due to evaporation. We study both processes by means of examples, beginning with steam.
We study a steam injection process as depicted in Fig. 19.8. A volume flow of moist air with dry- and wet-bulb temperatures T1 = 14 ◦C,