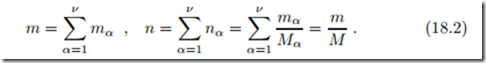Introduction
Many applications of thermodynamics involve not single substances but mixtures. The challenge is to track mixture composition, and to find the property data for the given composition. As long as mixture composition does not change, one can deal with mixtures the same way as with simple substances, including tabulating their properties; our treatment of air is the prime example of this.
Mixture composition can change through mixing or separation processes, through phase changes when the components have different vapor pressures, and through chemical reactions.
There is a vast array of applications for mixture theory, in particular in chemical engineering. Applications to be discussed include desalination of seawater, osmotic power plants, phase equilibrium and distillation processes, chemical equilibrium and NH3 production, and combustion.
In this and the following chapters we shall provide the tools to properly describe and evaluate these processes. The present chapter introduces additional properties to account for mixture composition, and relations between properties of components and the mixture as a whole.
Mixture Composition
We consider mixtures of ν components, indicated by greek subscripts α = 1, 2,... ,ν. The present chapter deals with non-reacting mixtures, reacting mixtures will be discussed later.
Throughout the following we assume that all components have the same temperature T . The mixture is contained in the volume V , and the mixing state is homogeneous, so that each component is equally distributed in V .
The composition of the mixture can either be described through the masses mα of the components contained in the volume V , or by their amount in molecule numbers Nα. Rather than tracking actual particle numbers, one uses the mole as a unit for counting particles, with the mole number defined as
Here ![]() Avogadro’s number, which defines the number of particles in one mole, and Mα is the molar mass, i.e., the mass of 1 mol of particles of type α.
Avogadro’s number, which defines the number of particles in one mole, and Mα is the molar mass, i.e., the mass of 1 mol of particles of type α.
The total mass m and the total mole number n of the mixture are obtained by summation over all components,
The last equation defines the average molar mass M of the mixture.
Often we will not be interested in the absolute amounts of the components, but in the relative amounts. Mass fraction cα (sometimes denoted as ”mass concentration”) and mole fraction Xα are defined as