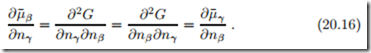Properties of the Chemical Potential
The Gibbs free energy G (T, p, nβ ) is an extensive quantity, which implies that it is a homogeneous function of mole number,
We take the derivative of the above with respect to z, to obtain
This must hold for all z. Since the left hand side is independent of z, the right hand side should not depend on z as well. This is so, when the chemical potential does not depend on all mole numbers nβ , but only on quotients like the mole ratio Xβ , which is independent of z, since Xβ (nγ ) = nβ , so that Xβ (znγ ) = Xβ (nγ ). Thus we have
For this reason, one sometimes finds the specific Gibbs free energy denoted as the chemical potential. For the description of the component, it is useful to distinguish between the Gibbs free energy g¯α (T, p) that describes the component α alone at (T, p) and the chemical potential μ¯α (T, p, Xβ ) that describes the component α in a mixture at (T, p) with mole fractions Xβ , β = 1,... , ν.
Since the order of derivatives can be exchanged, we have the symmetry property