Zeroth Law & Thermodynamic Equilibrium
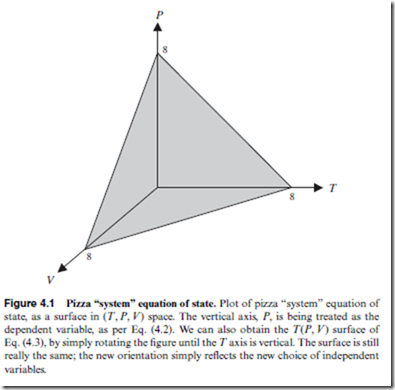
Equation of State
Although there are four thermodynamic variables in all (Section 3.1), under most circumstances, this set can be reduced down to just two independent variables. Throughout most of this book (until Chapter 15), the thermodynamic system of study is presumed to be closed, meaning that no particles can flow in or out. Consequently, N (or n) is a constant, rather than a variable. This reduces the set of thermodynamic variables down to three—i.e., T, P, and V .
Furthermore, for a system in thermodynamic equilibrium (Section 4.2), it has been established that only two of these three variables are independent. The third (dependent) variable is uniquely determined by the two independent variables, from which it can be obtained using the equation of state. This is a single equation involving all three variables, (T, P, V ), that constrains the allowed values of those variables—thereby specifying which thermodynamic states are in
All of the conclusions reached here regarding the pizza “system” have their analog in the thermodynamics world. In particular, every pure sub- stance is described by some equation of state that specifies which thermo- dynamic states are in equilibrium. The precise form of the equation of state depends on the particular substance—thereby accounting for many of the specific material properties of that substance.
Two important points should be kept in mind:
1. The equation of state refers only to systems in thermodynamic equilibrium. (The out-of-equilibrium case is discussed on pp. 26–27.)
2. The equation of state does not inherently treat any one thermodynamic variable differently than the other two.
Point 2 is particularly key, because it means that the choice of independent variables is flexible; it can be changed as desired, from one homework problem or laboratory experiment to the next. Thus, one might explicitly control T and V in one experiment, allowing the system to find its own P
as it achieves equilibrium; in another experiment, T and P might be fixed, in which case the system automatically expands or contracts until the equilibrium V value is reached.
By following the above Helpful Hint, you may avoid much of the confusion that often saddles beginning students of thermodynamics. A good choice of independent variables can also save you a lot of work. Remember, It’s OK to be Lazy.
Thermodynamic Equilibrium
Definition 4.1 A system in thermodynamic equilibrium is one for which (T, P, V ):
1. are well defined (meaning that T has the same value throughout the whole system, and that the same holds true for P).
2. remain constant over time (provided that external factors do not change).
This is a nice example of the one-to-many relationship between thermodynamic and molecular states.
Since the thermodynamic variables for a system in thermodynamic equilibrium are well defined and constant over time, the same must also be true of the thermodynamic state of that system.
This does not mean that the molecular state is constant over time—which would imply that the individual molecules are “frozen in place.” This is far from the case; at the molecular scale, particles are constantly moving, colliding, etc. But when the system is in thermodynamic equilibrium, all of these individual molecular changes tend to cancel each other out, statistically speaking, so that there is no net change at the macroscopic scale. Referring to Figure 3.1, one can imagine the system bouncing around from one point in the right plot (i.e., one molecular state) to another, but always within the region indicated. The corresponding thermodynamic state (the point indicated in the left plot) remains unchanged.
One can also imagine a different set of circumstances, under which the molecular state might change so much that it ends up moving outside of the Figure 3.1 (right plot) region. In this case, the final molecular state corresponds to a new thermodynamic state as well (i.e., a new point in the left plot). Such a macroscopic change of state can only occur as the result of the system somehow getting out of equilibrium.
Thus, when the macroscopic notion of “wind velocity” is analyzed at the molecular scale, it is actually found to have more to do with the number of air molecules moving in a preferred direction, than with their speeds. It would therefore be more accurate to refer to a “big” wind than to a “strong” or “fast” wind.
Zeroth Law
By virtue of Definition 4.1 (p. 26), together with the definition of the equa- tion of state (p. 23), there are two ways in which a system can be out of equilibrium:
derived from the Chinese and Japanese for “big wind.”
1. (T, P, V ) are well defined (same throughout system), but do not lie on the equation of state.
2. T and/or P vary from one point to another within the system.
Whenever a system gets out of equilibrium—however this has occurred— it undergoes a spontaneous macroscopic change, until a new equilibrium
Situation 2 above is often observed when the system consists of two subsystems—each initially in an equilibrium state, but not the same equilibrium state (i.e., different T and/or P values). When the two subsystems, A and B, are suddenly brought into contact and allowed to interact, they form one big system that is no longer in equilibrium. A spontaneous change then ensues, until the combined system achieves equilibrium. At this point, both subsystems have the same T and P throughout—but these values are different than what either subsystem started with.
Now imagine that in the above subsystems example, A and B start out with the same T and P values. Now when they are brought together, noth- ing happens; the combined system is already in thermodynamic equilib- rium, because T and P values are the same throughout. So in this case, there is no macroscopic change, and we say that A and B are in equilibrium with each other. Treating T and P as the independent variables, we also see that A and B are in the same thermodynamic state. This leads to the
Zeroth Law: If A is in equilibrium with B, and B is in equilibrium with C, then A is in equilibrium with C.
What is the real significance of this law? It simply confirms the existence of thermodynamic states, and the macroscopic completeness of thermody-thermometers work. namics.
We have discussed the idea of bringing subsystems into “contact,” but actually more than two, but only two for closed systems comprised of a pure substance in a single phase…
but for closed systems, the movable wall must still not allow particles through…
have not yet specified how this is done. It turns out that there are two kinds of contact, each associated with a different kind of equilibrium, and a different intensive thermodynamic variable.
Mechanical contact is associated with P, and with mechanical equilibrium. To bring two subsystems into mechanical contact, the wall that divides them must be allowed to move. Imagine that there is gas on either side of the dividing wall, initially at two different pressures, PA > PB. The pressure difference exerts a macroscopic force, F , tending to push the wall towards B, the low-pressure side:
Thus, if A and B are brought into mechanical contact, so that the fixed wall is suddenly allowed to move, it will do so—expanding subsystem A, com- pressing subsystem B, and transferring energy from A to B in the form of work—until finally, PA = PB = P, and mechanical equilibrium is restored.
Thermal contact is associated with T, and with thermal equilibrium.
Here, the dividing wall must be diathermic, or thermally conductive, in order for thermodynamic change to occur. If two subsystems are suddenly brought into thermal contact, and there is a nonzero temperature difference [(TA − TB) > 0], then energy will flow in the form of heat from the hotter subsystem (A) to the cooler one (B)—until TA equilibrium is restored.
= TB = T, and thermal Most real-world systems consist of more than one substance and/or phase of matter (e.g., gas, liquid, solid). In such cases—and also for open systems (the opposite of “closed”)—another form of contact known as diffusive contact also plays a key role. Analogs exist in chemical reactions, electrochemistry, and various other applications. Diffusive contact is discussed in Chapter 15; applications for which it is relevant are discussed in Chapter 17 and on the website.
Ideal Gases & Non-ideal Systems
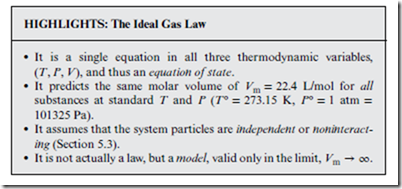
No thermodynamics book would be complete without at least a mention of the ideal gas law,
However, since it is no doubt already covered extensively in your primary textbook, along with non-ideal systems, we limit discussion here to just a few highlights.
Note that the ideal gas equation of state is the same for all substances. In reality—i.e., for non-ideal gases and condensed phases (liquids and solids)—the actual equation of state varies considerably from one sub- stance to another. In general, the ideal gas law breaks down as T → 0, because it predicts that the molar volume Vm → 0 in this limit—i.e.,
that the system becomes vanishingly small as it approaches absolute zero temperature—which is physically incorrect.
What in fact happens when T becomes small is that the system under- goes a phase transition to a condensed phase. The latter has a very small— but nevertheless nonzero—Vm value, which is also highly substance-specific (as is the transition temperature). Consequently, whereas the ideal gas law is usually a good approximation for real gases, it is completely invalid for condensed phases.
It is useful to characterize real substances in terms of the compressibility factor:
For ideal gases, Z = 1. For other materials, the sign and magnitude of (Z − 1) provide important information about the character and extent, respectively, of the non-ideality (see Sections 5.3, 9.3, and 15.3).
Much like n and N, the gas constant R and the Boltzmann constant k are macroscopic and molecular versions of the same quantity—being essentially, a conversion factor between temperature and energy (per mole for R; per particle for k). Thus,