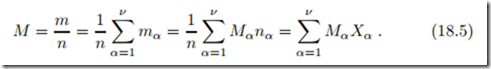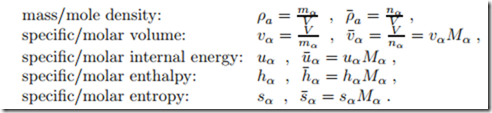Example: Composition and Molar Mass of Air
The average molar mass of a mixture is given by
Air is a mixture of several gases, the main components and their mole frac- tions and molar masses are
Accordingly, the average molar mass of air is Mair = 28.97 kg . The corresponding mass fractions are
Mixture Properties
In previous chapters, we have mainly used specific properties, that is proper- ties per unit mass which are denoted as, e.g., vα, uα, hα, sα. For mixtures it is often more convenient do refer to particle numbers, and thus we will often use mole based properties, denoted as, e.g., v¯α, u¯α, h¯α, s¯α.
Mole and mass based quantities are related through the molar mass Mα, in particular we have
Properties of the mixture are obtained as weighted sums over the properties of the individual components. We study this for the total internal energy, for which we have
Above, we have not indicated the dependencies between properties. In general, the properties of one component will depend on the presence of all other components. For instance, the internal energy of component α will depend on temperature T and total pressure p of the mixture, and on all mole fractions Xβ , β = 1,... ,ν, that is u¯α = u¯α (T, p, Xβ ). Therefore tabulated data for single components (where Xα = 1 and Xβ = 0 for β /= α) normally cannot be used. As will be seen, tabulated data for pure components can only be used for ideal gas mixtures, and ideal mixtures.
While all components have the same temperature T , they contribute to pressure differently. The partial pressure pα is the contribution of component α to total pressure p, with
Note that, in general, pα = pα (T, p, Xβ ), that is the partial pressure of a component will depend on the state and composition of the mixture.