Example: NH3 Production (Haber-Bosch Process)
Ammonia (NH3) is one of the most important materials in chemical industry. It is used for the production of fertilizers and explosives, as cooling fluid in refrigeration systems, and as source material for many other chemical processes.
The world ammonia production is about 130 × 106 t , and an ammonia plant that produces 1500 t consumes about 650 MW of energy; the energy required to produce one ton is ca. 35 GJ. Approximately 1% of the world’s energy usage is devoted to the production of ammonia!
Before Haber and Bosch found out how to produce ammonia industrially, the supply came from guano fields off the cost of South America (guano is . . . bird droppings). Here, we discuss the basic principles of the Haber-Bosch process which was developed by Fritz Haber (1868-1934) and brought to industrial production by Carl Bosch (1874-1940).
Ammonia is produced by combining hydrogen, normally obtained from natural gas by steam methane reforming, with nitrogen from air, where the oxygen is removed by reaction with carbon monoxide and hydrogen from the hydrogen production step. The chemical equation for ammonia synthesis reads
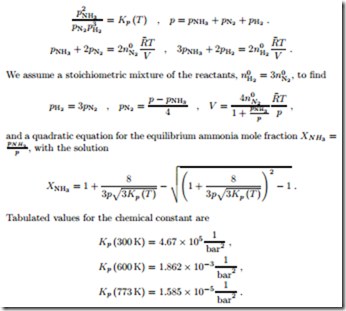
Thus, a larger ammonia fraction is encountered for smaller temperatures, where Kp (T ) is larger, and for larger pressures. For instance, at T = 300 K and p = 1 bar, one finds XNH3 = 0.935 and this increases to XNH3 = 0.9970 when the pressure is increased to 500 bar.
However, when one mixes hydrogen and nitrogen at (300 K, 1 bar), the mixture does not approach thermodynamic equilibrium, but remains in a metastable state; nothing happens, since the reaction rate is too slow. As Haber found out, iron catalysts are required to advance the reaction, but these work only at relatively high temperatures, where the ammonia yield is relatively small. In order to have a significant yield, the process must be performed under high pressures. For a reactor temperature of T = 773 K, the mole fraction is XNH3 = 0.309 for p = 500 bar, but only XNH3 = 0.0013 for p = 1 bar.
In continuous reactors, the product is cooled (at pressures below psat), the ammonia condenses and is removed, while the unused portions of hydrogen and nitrogen are fed back into the production process.