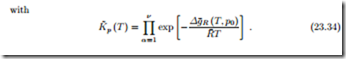Law of Mass Action
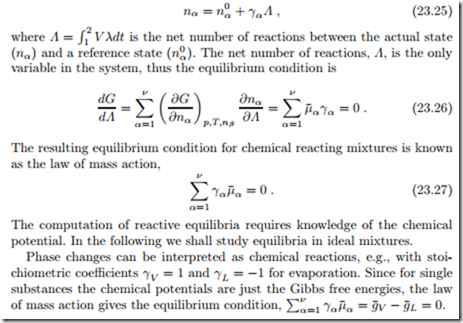
In equilibrium at constant pressure and temperature, the Gibbs free energy assumes a minimum, G (T, p, nα) −→ min. In a chemical reaction, the mole numbers nα are related by stoichiometry. From the mole balance (23.8) fol- lows
Law of Mass Action for Ideal Mixtures and Ideal Gases
For ideal mixtures the chemical potential is given by R¯T ln Xα, and the law of mass action gives
Related posts:
ICE MAKER AND REFRIGERATION CONTROLS:OPERATION OF THE CIRCUIT
Drive motors:Three phase electrical connections
Second Law & Spontaneous Irreversible Change
Coal Furnaces,Wood Furnaces, and Multi-Fuel Furnaces:Installation, Operating, and Maintenance Instru...
Heating Swimming Pools:Classifying Pool Heaters
Ice Maker and Refrigeration Controls:Household Ice Makers
Ventilation Principles:Components of a Roof Ventilator
Thermodynamics of Fuel Cells:Fuel Cell Potential
Air-Conditioning Equipment:Silver-Brazing Repairs