The Kelvin-Planck Statement
Even the—fully reversible—Carnot engine has a thermal efficiency ηC below unity: Not all heat received from the hot reservoir can be converted into work, some heat must be rejected to a colder reservoir. The Kelvin-Planck formulation of the second law states this as follows:
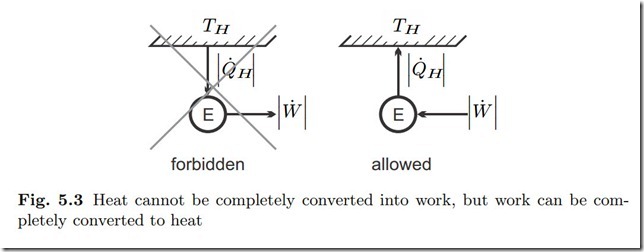
No steady state thermodynamic process is possible in which heat is completely converted into work.
This statement is a direct consequence of the first and second law. For a steady state process with just one heat exchange the laws require
hence heat and work must both be negative. Figure 5.3 shows the forbidden process, and also the—allowed—inverse process, the complete conversion of work into heat through friction. A typical example for the latter are resistance heaters in which electrical work is converted to heat through electric resistance.