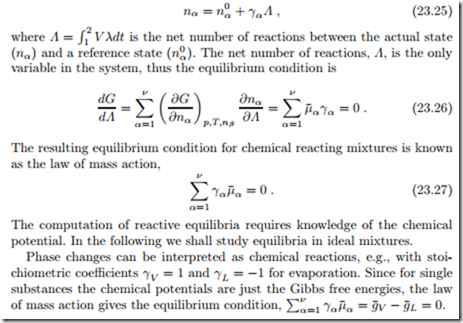
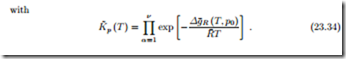Law of Mass Action
In equilibrium at constant pressure and temperature, the Gibbs free energy assumes a minimum, G (T, p, nα) −→ min. In a chemical reaction, the mole numbers nα are related by stoichiometry. From the mole balance (23.8) fol- lows
Law of Mass Action for Ideal Mixtures and Ideal Gases
For ideal mixtures the chemical potential is given by R¯T ln Xα, and the law of mass action gives
Related posts:
Electric Heating Systems:Baseboard Heating Systems
DUCTWORK SIZING:ACOUSTICAL TREATMENT
Steam and Hot-Water Space Heating Boilers:Boiler Operation, Service, and Maintenance
Hot-Water Heating Systems
Multiple Zone Air Systems:Dual Path Outside Air Systems
Single Zone Air Handlers and Unitary Equipment:Split Systems
Problems on Reversible Processes in Closed Systems
Heat Pumps:High-Pressure Switch and Low-Pressure Switch
Hydronic Heating Systems:Gravity Hot-Water Heating Systems