Secondary cells based upon aqueous electrolytes
Until the last few years of the twentieth century, the main forms of secondary or rechargeable battery were lead acid and nickel cadmium, both of which have been in use for about 100 years. Whilst lead acid has largely retained its market, nickel cadmium has been largely superseded by nickel metal hydride and lithium ion batteries. The latter, along with lithium batteries dominates a host of new consumer applications. Lithium cells will be treated separately as their construction and design are significantly different from the aqueous-based systems; however, nickel metal hydrides are similar in construction to nickel cadmium batteries.
Nickel cadmium and lead acid batteries are formed by connecting a number of cells in series. Each cell consists of vertical plates which are connected in parallel and are divided into a positive group and a negative group. Adjacent positive and negative
plates are insulated from each other by separators or rod insulators which have to provide the following functions:
● obstruct the transfer of ions between the plates as little as possible
● keep in place the active material
● enable the escape of charging gases from the electrolyte
All the plates and separators together form a plategroup or element.
The insulator in nickel cadmium batteries is formed from vertical plastic rods, which may be separate or as grids which are inserted between the plates. Corrugated or perforated PVC is also used as a separator. The active material is kept in place by steel pockets.
In lead acid batteries, the separators are normally microporous sheets of plastic, which are usually combined with corrugated and perforated spacers; porous rubber separators are also used. In some designs, the separator completely surrounds the positive or negative plate. The selection of separator is very important, and it depends upon the plate design and the use for which the battery is intended. In batteries intended for short discharges, such as the starting of the diesel engine, thin separators and spacers are used, whereas batteries designed for long discharges, as in standby power or emergency lighting, have thick separators and spacers. Batteries that require extra strength to withstand mechanical stresses often have a glass fibre separator against the positive plates in order to minimize the shedding of active material from these plates.
The outermost plates are of the same polarity on both sides. In lead acid batteries the outside plates are normally negative; in nickel cadmium batteries they are normally positive.
The plategroups are mounted in containers which are filled with electrolyte. The electrolyte is dilute sulphuric acid in lead acid batteries, and potassium hydroxide in nickel cadmium batteries. In a fully charged lead acid battery the electrolyte density is between 1.20 and 1.29. In nickel cadmium batteries it is between 1.18 and 1.30 irrespective of the state of charge.
The containers may house a single cell or they may be of a monoblock type, housing several cells. The top (or lid) may be glued or welded to the container, or it may be formed integrally with the container. In the lid is a hole for the vent, which is normally flame arresting, and the cells are topped up with electrolyte through the vent hole.
Below the plate there is an empty space which is known as the sludge space. This is provided so that shredded active material and corrosion product from the supporting structure can be deposited without creating any short circuits between the plates. The sludge space in nickel cadmium batteries is smaller than that in lead acid units because there is no corrosion of the supporting steel structure and no shredding of active material; it is usually created by hanging the plategroup from the lid. In lead acid batteries the sludge space may be created by hanging the plates from the lid or container walls (this is common in stationary batteries), by standing the plates on supports from the bottom (this is common for locomotive starting batteries) or by hanging the positive plates with the negative plates standing on supports (this is also used for stationary batteries).
From the plates, the current is carried through plate lugs, a connection strap or bridge and a polebolt. The bolts pass through the lid and are sealed against the lid with gaskets. Above the lid, the cells are connected to form the battery. Monoblocks may have connectors arranged directly through the side of the partition cell wall, in which case the polebolts do not pass through the lid, or the connectors may be located between the cell lid and an outer lid. The monoblocks made in this way are completely insulated and easy to keep clean.
The main forms of aqueous electrolyte secondary cell now commercially avail- able are:
● nickel cadmium – sealed
● nickel cadmium – vented
● nickel metal hydride
● lead acid – pasted plate
● lead acid – tubular
● lead acid – Planté
● lead acid – valve-regulated sealed (VRSLA)
Each of these is described separately in sections 12.3.1 to 12.3.7, and a summary of the main features of the four lead acid types and nickel cadmium is given in Table 12.1.
Sealed nickel cadmium
This type utilizes a cadmium positive and a nickel oxyhydroxide negative with alkaline electrolyte and has been commercially available since the early 1950s. The single cell operates across a voltage range 1.0 V to 1.25 V and is ideally suited to a heavy continuous current drain of up to eight times the nominal ampere-hour capacity of the battery. The low internal resistance of the cell makes it ideal for heavy current discharge applications in motor-driven appliances such as portable drills, vacuum cleaners, toys and emergency systems. Batteries of this type are capable of being recharged hundreds of times using a simple constant current charging method; however the electrochemistry has a memory effect and capacity is progressively lost if the batteries are completely discharged and then fully recharged.
The cells tolerate a wide temperature range from –30°C to 50°C, and they have a shelf life of up to eight years at 20°C. Typical construction of a sealed nickel cadmium cell is shown in Fig. 12.9. The cell is available commercially in a variety of standard sizes including AAA, AA, C, D, PP3 and PP9.
Vented nickel cadmium
Using the same electrochemistry as the sealed variant, vented cells use ‘pocket’ plates, which are made from finely perforated nickel-plated steel strip filled with active material. The pocket plates are crimped together to produce a homogenous plate. Translucent plastic or stainless steel containers are used. An example is shown in Fig. 12.10.
The batteries are robust and they have the benefits of all-round reliability; they are resistant to shock and extremes of temperature and electrical loading. They can be left discharged without damage and require very little maintenance. Their cycling ability is excellent and can offer a service life of up to 25 years.
Vented nickel cadmium batteries are used typically in long-life applications where reliability through temperature extremes is required and where physical or electrical abuse is likely. These applications include railway rolling stock, off-shore use, power
system switch tripping and closing, telecommunications, uninterruptible power supplies, security and emergency systems and engine starting.
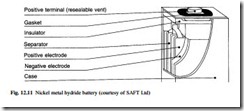
Nickel metal hydride
The sealed nickel metal hydride battery has become commercially available in the past few years and market share has rapidly risen to make this one of the major technologies. A single cell operates with a voltage range of 1.1 V to 1.45 V, and a nominal voltage of 1.2 V. The construction of a metal hydride cell is illustrated in Fig. 12.11.
These batteries are relatively expensive, but they have no toxicity problems on recycling. Their energy density is in the region of 60–70 Wh/kg, which is higher than the sealed nickel cadmium battery. The operating temperature range is –20°C to 60°C
and the shelf life is up to five years. Nickel metal hydride batteries are suitable for applications with milliampere current drains from 0.2 to 3 times the nominal ampere- hour capacity of the battery. The high energy density and specific power (perhaps 250 W kg−1) makes this type of battery ideally suited to applications for memory back-up and portable communications. Nickel metal hydride batteries will endure recharging up to 500 times, although the method of charging is slightly more complicated than for sealed nickel cadmium systems.
Lead acid – pasted plate
The positive plates of pasted plate cells are made by impressing an oxide paste into a current-collecting lead alloy grid, in which the paste then forms the active material of the plate. The paste is held in position by a long interlocking grid section. In addition to the long-life microporous plastic separator, a glass-fibre mat is used. This becomes embedded in the face of the positive plate holding in place the active material and so prolongs the life of the battery.
Cells can be manufactured with lead–antimony–selenium alloy plates. These require very little maintenance, offer good cycling performance and are tolerant to elevated temperatures. Alternatively, lead–calcium–tin alloy plates may be used; these offer even lower maintenance levels.
Pasted-plate lead acid batteries are the lowest in purchase costs; their service life is in the range 2 to 20 years and the maintenance requirements are low, with extended intervals between watering. They are compact and provide high power density with excellent power output for short durations. Typical applications are in medium-to-long duration uses where initial capital cost is a primary consideration. These include telecommunications, UPS, power generation transmission, switch tripping and closing, emergency lighting and engine starting.
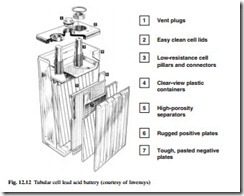
Lead acid – tubular
In a tubular cell, an example of which is shown in Fig. 12.12, the positive plate is constructed from a series of vertical lead alloy spines or fingers which resemble a comb. The active material is lead oxide; this is packed around each spine and is retained by tubes of woven glass fibre which are protected by an outer sleeve of woven polyester or perforated PVC. This plate design enables the cell to withstand the frequent charge–discharge cycles which cause rapid deterioration in other types of lead acid cell.
The advantages of the tubular cell are excellent deep cycling characteristics, service life of up to 15 years and a compact layout which gives a high power per unit volume. The low antimony types also offer extended watering intervals.
Typical applications are where the power supply is unreliable, and where discharges are likely to be both frequent and deep. These include telecommunications, UPS, emergency lighting and solar energy.
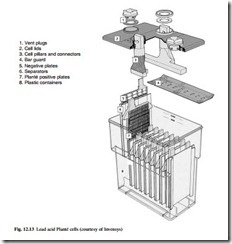
Lead acid – Planté
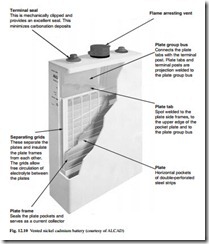
The distinguishing feature of the Planté cell is the single pure lead casting of the Planté positive plate. The active material is formed from the plate surface, eliminating the need for mechanical bonding of a separately applied active material to a current collector. This makes the Planté cell the most reliable of all lead acid types. An example of the construction of Planté batteries is shown in Fig. 12.13.
Ultra High Performance Planté cells have a thinner plate. This gives the highest standards of reliability for UPS and other high-rate applications. The advantages of the Planté cell are extremely long life (which can be in excess of 20 years for High Performance cells and 15 years for Ultra High Performance cells) and the highest levels of reliability and integrity. Constant capacity is available throughout the service life, an assessment of condition and residual life can readily be made by visual inspection. Usage at high temperatures can be tolerated without significantly compromising the life expectancy, and maintenance requirements are very low.
Typical applications are long-life float duties in which the ultimate in reliability is required. These include critical power station systems, telecommunications, UPS, switch tripping and closing, emergency lighting and engine starting.
Lead acid – valve regulated sealed (VRSLA)
In sealed designs, lead calcium or pure lead is used in the grids. The separator is a vital part of the design because of its influence on gas recombination, and the amount of electrolyte that it retains; it often consists of a highly porous sheet of microfibre. Examples of sealed cells are shown in Fig. 12.14.
Besides these sealed units, there is a large variety of so-called maintenance-free batteries which rely more upon carefully controlled charging than upon the gas recombination mechanism within the cells. Non-antimonial grids and pasted plates are used, and in most units the electrolyte is immobilized or gelled. The batteries are provided with vents, which open at a relatively low overpressure.
The benefits are a long life, no topping up, no acid fumes and no requirement for forced ventilation. There is no need for a separate battery room and the units can be located within the enclosure of an electronic system. In addition, these batteries are lighter and smaller than the traditional vented cells.
Typical applications include main exchanges for telecommunications, PABX systems, cellular radio, microwave links, UPS, switch tripping and closing, emergency lighting and engine starting.