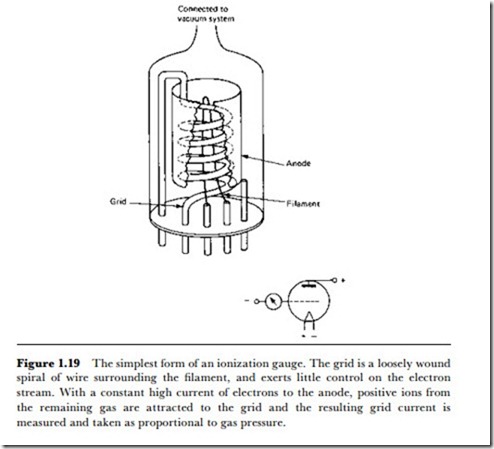
Ionization gauges
For very low pressure, or high vacuum, measurement, some form of ionization gauge is invariably used. There are many gauges of this type, but the principles are much the same and the differences are easily understood when the principles are grasped. The ionization gauge operates by using a stream of electrons to ionize a sample of the remaining gas in the space in which the pressure is being measured. The positive gas ions are then attracted to a negatively charged electrode, and the amount of current carried by these ions is measured. Since the number of ions per unit volume depends on the number of atoms per unit volume, and this latter figure depends on pressure, the reading of ion current should be reasonably proportional to gas pressure. The proportionality is fairly constant for a fixed geometry of the gauge (Figure 1.19) and for a constant level of electron emission. The range of the gauge is to about 10-7 mm (0.013 Pa), which is about the pressure used in pumping transmitting radio valves and specialized cathode ray tubes.
The most serious problem in using an ionization gauge is that it requires electron emission into a space that is not a perfect vacuum. The type of electron emitter that is used in the hot-cathode or Bayard-Alpert gauge is invariably a tungsten filament. If this is heated at any time when the gas pressure is too high (above 10-3 mm, 133 Pa), then the filament will be adversely affected. If, as is usual, the gas whose pressure is being reduced is air, the operation of the filament at these pressures will result in oxidation, which will impair electron emission or result in the total burnout of the filament. If hot-cathode ionization gauges are used, as they nearly always are, in conjunction with other gauges, usually Pirani gauges, then it should be possible to interlock the supplies so that the ionization gauge cannot be turned on until the pressure as indicated by the other gauge, is sufficiently low. If this can be done, then the ionization gauge can have a long and useful life. A spare gauge head should always be held in stock, however, in case of filament damage, because tungsten filaments are delicate, particularly when at full working temperature. Each gauge head will
need to be calibrated if precise measurements of low pressure are required.
A common variation on the ionization method is the Penning gauge, which uses electron emission from a point (a cold-cathode emitter). This avoids cathode damage from oxidation and from fluorine, and the same advantage is claimed for ionization gauges that use thoria-coated iridium (ThOIr) cathodes. A tungsten filament is not poisoned by halogen gases, and is preferred for applications that involve fluorine, chlorine or iodine gases.
Other variants on the ionization gauge arise because a simple electron beam in a confined space is not necessarily a very efficient means of ionizing the residual gas in that space, because only the atoms in the path of the beam can be affected. If the electron beam is taken through a longer path, more atoms can be bombarded, and more ions generated from a given volume of gas, and so the sensitivity of the device is greatly increased. The usual scheme is to use a magnetic field to convert the normal straight path of the electron beam into a spiral path that can be of a much greater total length. This is the magnetron principle, used in the magnetron tube to generate microwave frequencies by spinning electrons into a circular path that just touches a metal cavity, so that the cavity resonates and so modulates the electron beam.
The much greater sensitivity that can be obtained in this way is bought at the price of having another parameter, the magnetic field flux density, that will have to be controlled in order to ensure that correct calibration is maintained. The magnetic field is usually applied by means of a permanent magnet, so that day-to-day calibration is good, but since all permanent magnets lose field strength over a long period, the calibration should be checked annually. Gauges of this type can be used down to very low pressures, of the order of 10-11 Pa.
• On the other end of the pressure range, a radioactive material can be used as a source of ionization, and this allows measurements up to much higher ranges of pressure, typically up to 105 Pa.