INTRODUCTION
You don’t have to be an engineer, physicist, or mathematical genius to learn electronics. But there are a few basic things that you need to know so you can understand what is going on in the circuits and equipment. For example, you need to know that electrons are what makes up current flow, and voltage is what makes the current flow. You also need to know about magnetic fields and electric fields, where they come from, and what they do. And how magnetism and current flow are related. With that background you are ready to learn about the various components and circuits. This chapter takes care of those basics.
ELECTRICITY AND ELECTRONICS
Electricity is a type of energy produced by a charge that is either fixed (static) or moving (dynamic). The main source of electricity is the electron, a sub- atomic particle that has a negative charge. When electrons are stored or moved, electricity is produced.
Electricity is one of our prime sources of energy. It is used for lighting, heating, and operating appliances of all sorts. It powers motors to produce mechanical energy, and it powers our electronic equipment. The uses of electricity are virtually infinite.
Electronics is the field of applied science that uses components such as resistors, capacitors, diodes, transistors, and integrated circuits to control and process electricity. These components are used to create circuits that convert, modify, vary, translate, or otherwise manipulate electrical charges to perform useful functions. That is the essence of it. Now for the details.
Atoms and Electrons
This section really ought to be called Physics 101 but I don’t want to scare you away. Just to make you more comfortable, I have to say that it is not all that complicated or difficult to understand. So even if you avoided physics in high school, just be aware that it is pretty easy. Trust me.
All matter, whether it is a solid, a liquid, or gas, is made up of tiny entities called atoms. An atom is the smallest possible particle of substances called elements. An element is a chemical substance that cannot be subdivided into smaller, simpler substances. Typical elements are hydrogen, oxygen, gold, silver, copper, carbon, helium, silicon, and sodium. All matter is either an element or composed of several elements. Atoms of two or more elements often com- bine to form new substances called compounds. For example, water is a com- pound of hydrogen (H) and oxygen (O) or H2 O. Salt is a compound of sodium (Na) and chlorine (Cl) or NaCl. The smallest possible particle of a compound that exhibits all the characteristics of the substance is called a molecule.
According to the theories of physics, an atom is composed of a nucleus consisting of a core of tightly bound subatomic particles, called protons and neutrons. Protons have a positive charge. Neutrons are neutral, having neither a positive nor a negative charge.
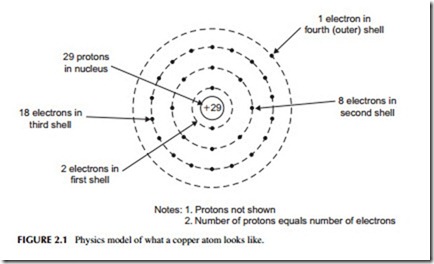
Rotating around the nucleus are electrons. The electrons arrange them- selves in multiple orbits in much the same way that the planets orbit around the sun. The orbits are sometimes referred to as rings or shells. Electrons have a negative charge. Figure 2.1 illustrates a copper atom. The number of electrons in orbit equals the number of protons in the nucleus. The number of positive charges equals the number of negative charges. Therefore, the atom is balanced electrically.
As far as electronics is concerned, the most important part of an atom is its electrons. Since electrons can be manipulated (i.e., stored or moved), they can be used to produce electricity. This electricity can then, in turn, be processed and controlled to perform a wide range of valuable functions. By applying an external force, the electrons in the outer shells can be stripped off to produce current flow.
Charge, Voltage, and Current
There are two basic types of electricity, static and dynamic. Static electricity is the buildup of a charge between two objects. Dynamic electricity supplies a continuous charge.
With static electricity, one object has an excess of electrons and the other has a shortage of electrons. The object with an excess of electrons is therefore nega- tive while the object with a shortage of electrons is positive. A basic law of electricity states that opposite charges attract and like charges repel. The two oppositely charged bodies, one negative and one positive, will have a high physical attraction for one another. An invisible force field called an electric field will exist between the two charged objects. The object with a shortage of electrons attracts the object with excess electrons.
The two oppositely charged objects will naturally attract one another. The closer they are, the greater the attraction. This attraction of the charges sets up an electrical force field between the two bodies. This electric field, like a magnetic field, contains energy that can be released to perform useful work.
Whenever there are opposite charges on two objects, we say that an electrical potential or difference of potential exists between them. This difference of potential is called voltage. Voltage is an electrical pressure that can cause work to be accomplished.
The charge stored on the two objects is usually called static electricity because it is stationary. However, this charge is usually dissipated in some way. If the two charged objects get too close, or the charge builds up too high, the attractive force becomes so great that the electrons jump the gap between them and create a spark. That is how lightning occurs. The clouds and the earth become oppositely charged, the earth positive, the clouds negative. The charge becomes so great that the electrons leap across the free space gap, ionizing the air and creating a path through which the electrons travel to neutralize the positive charge on the earth. The result is the massive spark or flash that we call lightning and the roar we call thunder.
In general, static electricity is not very useful. It represents energy stored as a charge, but it quickly dissipates as a spark or a brief flow of electrons between the negatively and positively charged objects when the two charged objects get close enough or when the charge gets too high. We call this an electrostatic dis- charge (ESD). Static electricity, or ESD, is regarded as more of a disadvantage or nuisance than anything else. In fact, it is one of the main causes of damage to electronic components such as transistors and integrated circuits.
Neutralizing a charge between two objects cancels or eliminates the potential. However, if we had a constant charge that could constantly be replenished, we would have a continual source of potential (voltage) and could therefore sustain a flow of electrons. With such a source of electrical energy, useful work can be accomplished.
Dynamic Electricity and Current Flow
Electricity as we know it, whether in electrical power applications or in electronics, is actually the flow of electrons. Electrons flowing from one place to another is known as current flow. And it is voltage that causes the current to flow. When electrons flow, we have energy that can be put to practical use. Electrons flowing in a filament create light. Electrons flowing in a heating coil produce heat. Electrons flowing in a motor produce mechanical energy. The object is to convert the energy from one form to another. In electronics, we precisely control the electrons with special components and circuits to produce a variety of effects.
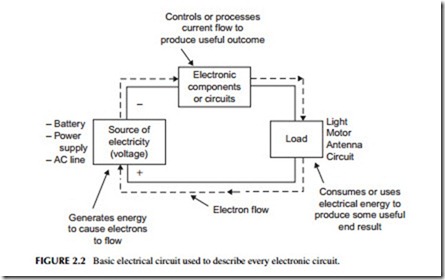
Figure 2.2 provides a general block diagram model that shows how all electrical or electronic circuits work. A voltage source produces the energy that causes electrons to flow. This current flow is controlled or processed by a single electronic component, or many components that make up an electronic circuit. The processed electrons flow through a load that consumes or uses the electrical energy to produce a useful output. In an electrical application, the load may be a light, a heating element, or motor, as described above. In an electronic application, the load may be an antenna that radiates the radio signal produced by the electronic circuits. Or the load may be the magnetic head that develops and records digital data on a hard disk or a laser that records a video signal on a DVD.
A couple of key facts about the circuit in Figure 2.2 are required here. First, there must be a voltage source to cause the current to flow. The electrons flow from the negative terminal of the voltage source around the circuit as they are attracted by the positive terminal. Second, the circuit must always form a complete loop from plus to minus.
Current is so important that we need some way to measure it. We do this by standardizing the number of electrons that move past one point in a conductor
Conductors, Insulators, and Semiconductors
A conductor is a material that has many electrons easily freed up by an external voltage. Current flows through conductors easily. They have what we call low resistance. Most good conductors are metals like copper, silver, or aluminum, with loosely bound electrons that can be freed by an external volt- age to create current flow. Copper wire is the most commonly used conductor because of its low cost and its ability to be formed into many different shapes and sizes. Most metals are conductors but so is salt water.
Insulators are just the opposite of conductors. These are usually compounds in which the electrons are tightly bound together with the nuclei of the atoms. Even with lots of voltage applied, the electrons are hard to strip away to make current. Insulators keep current from flowing. Some common insulator materials are glass, ceramic, and plastics.
A third category of material is called semiconductor. It is a material that can be changed to make it a good conductor or a good insulator or anything in between. Semiconductors are used to make the transistors, diodes, and inte- grated circuits. The most common semiconductor is silicon. Others are germanium and carbon. Compounds such as gallium arsenide (GaAs), indium phosphide (InP), and silicon germanium (SiGe) are examples. Most integrated circuits (ICs) or chips are made of silicon.