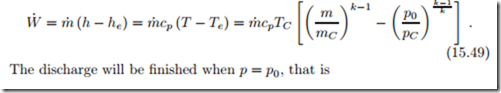Reversible Discharge after Cooling
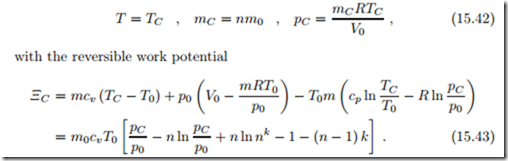
Real life compressed air storage will have a storage efficiency below 100% due to irreversible losses in compressor and turbine during filling and discharge, and due to energy loss by heat transfer from the hot compressed gas to the environment. To get some insight, we assume that, while filling and discharge happen adiabatically and reversibly, the container loses some heat during storage, so that the temperature drops to TC . In this case, the state of the air in the container is
Since pressure and temperature are lower than directly after filling, the work potential has dropped, ΞC < Ξn.
Realizing the work potential is another question. We study the inversion of the filling process, that is a turbine for the reversible discharge of the air into the environment. Again, we assume an adiabatic process with constant mass flow. During discharge, the state of the air in the container changes due to expansion. For this computation it is best to consider container and turbine separately. With the present state in the container denoted by T , p, u, s and so on, the discharge from the adiabatic container is given by mass and energy balance,
Interestingly, while the pressure ratio changes throughout the process, the turbine exit temperature remains at the same value.
The power provided by the turbine is
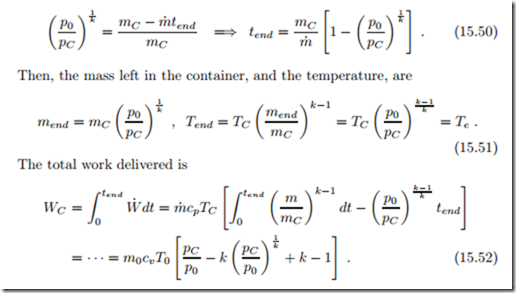
The pressure pC assumes values between the reversible filling pressure pn = p0nk and np0, which is the pressure when the compressed air is thermally equilibrated with the environment at T0.
When no heat loss occurs (TC = Tn), the turbine exit temperature is just
the environmental temperature, Te = Tn p0 n = T0, and it lies below
T0, when the air lost energy to the environment. In the extreme case that TC = T0, the turbine exit temperature is T0n k < T0. The actual work delivered is below the work potential (15.43), since the air leaving the turbine is colder than the environmental air, and thus there is work potential due to temperature difference between exhaust and environment. When the cold exhaust just mixes with the environmental air, entropy is produced, and this work potential is lost.
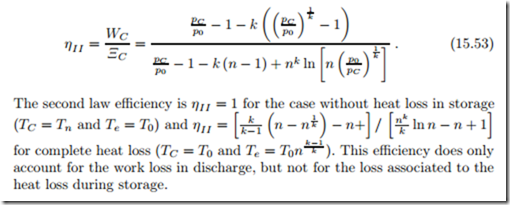
The second law efficiency for the discharge process alone is the ratio between the work produced and the initial work potential,
The storage efficiency for the complete filling and discharge process is the ratio between the work produced in discharge, and the work required for filling,