Fuel Cells
We have seen before that entropy generation in combustion, and subsequent heat transfer, leads to considerable irreversible work losses. The combustion loss can be attributed to the uncontrolled movement of electrons as new molecules are formed in the reaction. Fuel cells offer a process in which the electron movement is controlled, and thus no combustion losses occur. The performance of real life fuel cells is diminished by irreversible losses due to resistance, reaction activation and mass flow restrictions, and these will be discussed after the basic discussion of fuel cells and their efficiencies.
In fuel cells an electrochemical process allows to directly convert the energy stored in a fuel into electrical energy. There are many types of fuel cells that consume different fuels, e.g. direct methanol fuel cells, direct carbon fuel cells, and hydrogen fuel cells. Large parts of the discussion below are valid for all types, however, when it comes to evaluation of the equations, we shall consider only hydrogen fuel cells with the overall reaction
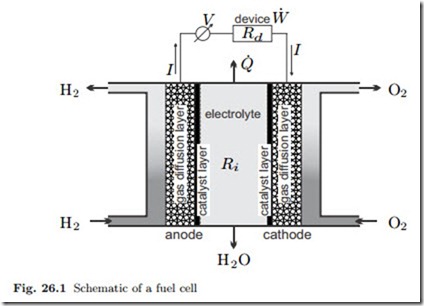
Figure 26.1 shows a schematic of a hydrogen fuel cell. Hydrogen and oxy- gen (pure or with air) are supplied to the two sides of the fuel cell in transport channels, and enter gas diffusion layers (GDL) through which they travel to- wards the catalyst layer. The GDL shields the gas from the electrolyte, and contributes to the management of water flows within the cell. The electro- chemical reactions take place in the catalyst layers at anode and cathode. These are separated by an electrolyte through which electrons cannot pass. The electrons move from anode to cathode through the electrical device (with resistance Rd) and provide the power W˙ ions move through the electrolyte.
to run the device. At the same time The reactions at anode and cathode, and the transport processes through the electrolyte depend on the type of electrolyte.
Figure 26.2 sketches the reactions for an acidic electrolyte, e.g., phosphoric acid (PO(3−) + 3H++ water), or polyelectrolyte membranes (PEM), which are polymers with sulfuric acid groups (polymer +SO− + H++ water). At the anode, the incoming hydrogen is split into electrons, e−, and protons, H+. The protons travel through the electrolyte to the cathode, while the electrons pass through the device. At the cathode, protons, electrons and the incoming oxygen react to water, which must be removed from the electrolyte.
In alkaline electrolyte fuel cells, see Fig. 26.3, the electrolyte is a base, e.g., potassium hydroxide (K+ + OH−+ water). At the anode, the incoming hydrogen reacts with hydroxide, OH−, to form water and electrons, which travel through the electrical device to the cathode. At the cathode, the electrons and the incoming oxygen react with water to form hydroxide, that then travels through the electrolyte to the anode, while half of the water produced at the anode travels through the electrolyte to replenish the water consumed in the cathode reaction.
In both types of fuel cells, the ion and electron movement is forced by the electric potential V between cathode and anode.